Clinical Genitourinary Cancer is a peer-reviewed journal that publishes original articles describing various aspects of clinical and translational research in genitourinary cancersClinical Genitourinary Cancer is devoted to articles on detection diagnosis prevention and treatment of genitourinary cancersThe main emphasis is on recent scientific developments in all areas related to. Higher toxicity regarding asthenia and gastrointestinal side effects along with a better.

Immunosuppressives may diminish therapeutic effects of vaccines and increase risk of adverse effects increased risk of infection.
Cisplatin adverse effects. Cisplatin decreases effects of adenovirus types 4 and 7 live oral by pharmacodynamic antagonism. Avoid or Use Alternate Drug. Immunosuppressives may diminish therapeutic effects of vaccines and increase risk of adverse effects increased risk of infection.
Live-attenuated vaccines should be avoided for at least 3mo after cessation of. Commonly reported side effects of cisplatin include. Bone marrow depression nephrotoxicity and ototoxicity.
See below for a comprehensive list of adverse effects. Intravenous powder for solution intravenous solution. Intravenous route Powder for Solution Nephrotoxicity.
Cisplatin injection can cause severe renal toxicity including. The authors have designed a randomized phase III controlled study comparing the efficacy of gemcitabine and cisplatin and dose-dense methotrexate vinblastine doxorubicin and cisplatin dd-MVAC in patients for whom chemotherapy has been decided before or after radical cystectomy. Higher toxicity regarding asthenia and gastrointestinal side effects along with a better.
Administration of gemcitabine-cisplatin the current standard therapy for advanced biliary tract cancers results in median progression-free survival and overall survival of 80 and 117 months respectively. New treatments offering improved survival outcomes are therefore needed. To evaluate the association between progression-free survival and the addition of.
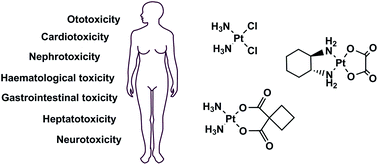
Cisplatin CP stimulates DNA damage and apoptosis in cancer chemotherapy. However CP has adverse effects on several organs of the body and drug resistance is frequently observed. The purpose of the present review is to show the function of curcumin in decreasing CPs adverse impacts and improving its antitumor activity.
Curcumin administration reduces ROS levels to prevent apoptosis. Gemcitabine is a chemotherapy drug that works by killing any cells that are dividing. Cancer cells divide rapidly and so are targeted at higher rates by gemcitabine but many essential cells also divide rapidly including cells in skin the scalp the stomach lining and bone marrow resulting in.
In clinical trials cisplatin is often selected due to its strong antitumor activity but its adverse effects include renal toxicity Iwasaki Nagata et al 2005 nausea and vomiting Kosmas Tsavaris et al 2001. Therefore to avoid renal toxicity urine volumes should be monitored and large-dose infusion is mandatory in cisplatin based chemotherapy. In clinical practice carboplatin has.
A phase III clinical trial reported superiority of cisplatinirinotecan over cisplatinetoposide in a Japanese population 209. Patients may also have treatment-related adverse effects such as pulmonary fibrosis or cardiac complications which may benefit from specialist input 221. The management of these patients by a multidisciplinary team that includes non-oncology specialists is.
Immunosuppressives may diminish therapeutic effects of vaccines and increase risk of adverse effects increased risk of infection. Live-attenuated vaccines should be avoided for at least 3mo after cessation of immunosuppressive therapy. Either increases toxicity of the other by pharmacodynamic synergism.
Avoid or Use Alternate Drug. Avoid use of. Both cisplatin and furosemide can cause nephrotoxicity and ototoxicity which may be additive when used together.
Use of dichlorphenamide and furosemide is not recommended because of an increased risk of furosemide-related adverse effects and risk for hypokalemia. Monitor closely for signs of drug toxicity if coadministration cannot be avoided in some patients furosemide dose adjustment. Clinical Genitourinary Cancer is a peer-reviewed journal that publishes original articles describing various aspects of clinical and translational research in genitourinary cancersClinical Genitourinary Cancer is devoted to articles on detection diagnosis prevention and treatment of genitourinary cancersThe main emphasis is on recent scientific developments in all areas related to.
The differences in potencies for carboplatin and cisplatin appear to be directly related to the difference in aquation rates. In patients with creatinine clearances of about 60 mLmin or greater plasma levels of intact carboplatin decay in a biphasic manner after a 30-minute intravenous infusion of 300 mgm2 to 500 mgm2 of carboplatin. The initial plasma half-life.
Beware of delayed emesis with Cisplatin. ADVERSE EFFECTS REGIMEN SPECIFIC COMPLICATIONS Diarrhoea mucositis steroid side effects nausea or vomiting. Nephrotoxicity - ensure adequate pre and post hydration is prescribed.
Ototoxicity - assess patient for tinnitus or hearing abnormalities. Severe cytokine release syndrome is characterised by severe dyspnoea often. The effects of chemotherapy should not interfere with the planned course of radiation and one advantage of cisplatin is that it has limited adverse effects on bone marrow.
In our study the rate. Safety and adverse events will also be evaluated. Cisplatin 75 mgm2 pemetrexed 500 mgm2 and nivolumab 360 mgbody were administered intravenously every 3 weeks with a total of 46 cycles.
If patients did not progress during the combination phase maintenance therapy with nivolumab was administered until disease progression or. This section needs expansion. The chemotherapy used was Cisplatin or Carboplatin combined with Pemetrexed or Paclitaxel.
The data were presented in an abstract format and as a lecture during the American Society of Clinical Oncology ASCO 2020 annual meeting. Median OS was 156 and 109 months in the immunotherapy-chemotherapy and the chemotherapy only groups. A consistent approach to the symptomatic treatment of adverse effects can considerably improve tolerance and consequently outcome.
Overview Basics of chemotherapeutic agents action 1 2 Kinetics. Chemotherapeutic agents are most active on cells with a high growth fraction ie cells actively undergoing division including normal cells such as epithelial or bone marrow cells as well as. SIDE EFFECTS Infusion-Related Events.
During or soon after rapid infusion of vancomycin patients may develop anaphylactoid reactions including hypotension see Animal Pharmacology wheezing dyspnea urticaria or pruritusRapid infusion may also cause flushing of the upper body red neck or pain and muscle spasm of the chest and back. The most common adverse reactions reported in greater than or equal to 4 of 300 adults receiving a single 24 mg dose of ondansetron orally in 2 trials for the prevention of nausea and vomiting associated with highly emetogenic chemotherapy cisplatin greater than or equal to 50 mgm 2 were. Headache 11 and diarrhea 4.
Serious adverse reactions Warnings and Precautions Serious and sometimes fatal adverse reactions with increased incidence in the Avastin-treated arm vs chemotherapy arm included. Gastrointestinal GI perforation ranged from 03 to 3 of patients across clinical studies. Non-GI fistulae.
The European Journal of Cancer EJC integrates preclinical translational and clinical research in cancer from epidemiology carcinogenesis and biology through to innovations in cancer treatment and patient careThe journal publishes original research reviews previews editorial comments and correspondence. The EJC is the official journal of the European Organisation for Research and.