A pentyl amine b methyl-n-propyl amine c diethyl amine d 2-aminopentane e isobutylamine 23. CeFAZolin cefOXitin cefTAZidime cefTRIAXone.
The alkoyl groups are derived from marine fatty acids C12-C24 25-Di-tert-amylhydroquinone Diamines derived from dimerized vegetable oil acids Diaryl-p-phenylenediamine where the aryl group may be phenyl tolyl or xylyl 12-Dibromo-24-dicyanobutane CAS Registry No.
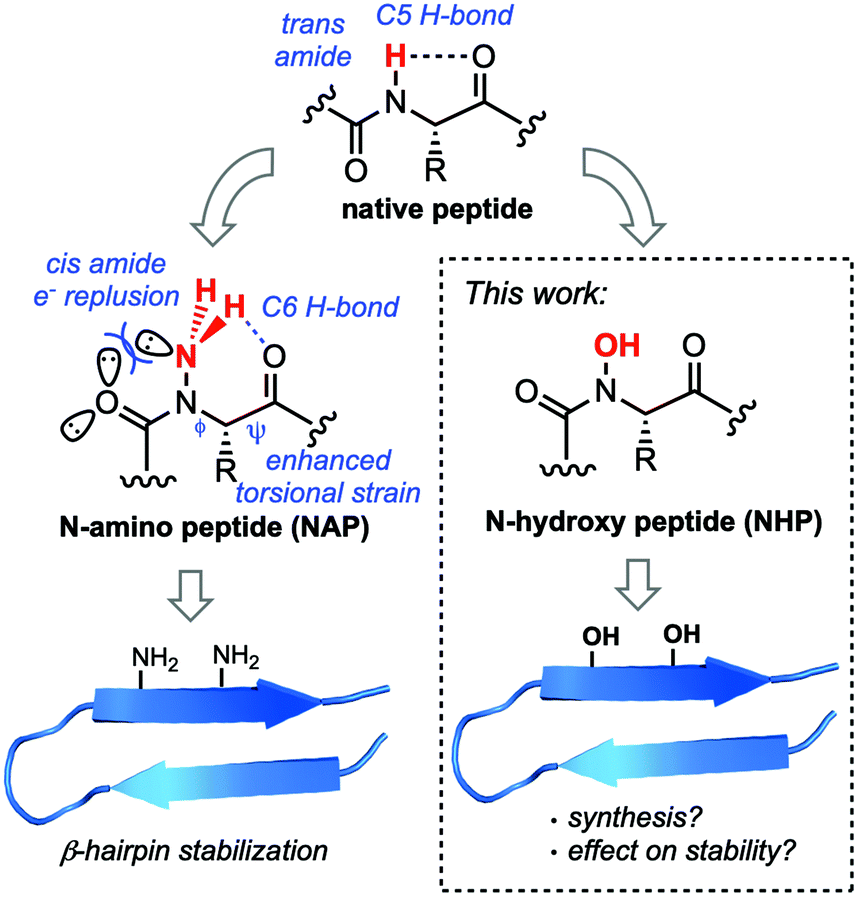
Cis n hydroxy amide. Peptide bonds to proline and to other N-substituted amino acids such as sarcosine are able to populate both the cis and trans isomers. Most peptide bonds overwhelmingly adopt the trans isomer typically 999 under unstrained conditions chiefly because the amide hydrogen trans isomer offers less steric repulsion to the preceding C α atom than does the following C α atom cis isomer. Arginine amide Aspartic acid 1-amide Asparagine amide Cysteine amide Glutamic acid 1-amide Glutamine amide Glycine amide Histidine amide Isoleucine amide Leucine amide Lysine amide Methionine amide Phenylalanine amide Proline amide Serine amide Threonine amide Tryptophan amide Tyrosine amide Valine amide.
Sterile outlet connection diam. 916 in pore size 02 μm pore size 05 μm cartridge nominal length 47 in. D primary amide e secondary amide 22.
The systematic name for the compound in Problem 21 is _____. A pentyl amine b methyl-n-propyl amine c diethyl amine d 2-aminopentane e isobutylamine 23. Select the IUPAC name for the compound below.
A 24-dimethylpentanoic acid b 113-trimethylbutanoic acid c 1-hydroxy-24. Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons. The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values.
M l 0 1 2. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Amide R NH 2 O amide carboxamide carbamoyl R-CONH2 nitrile R N nitrile cyano R-C㲇N aldehyde R H O al carbaldehyde oxo R-CHO ketone R R O one oxo R-CO-R alcohol ROH ol hydroxy R-OH amine RNH 2 amine amino R-NH2 ether R O R ether alkoxy R-O-R alkyl bromide alkyl halide RBr bromo halo R-Br R-X Nomenclature rules gjr–H3C CH3 CH3 4-methylheptane H3C CH3 OH 2.
When n is four or more isomers are possible distinguished by the position and conformation of the double bond. Alkenes are generally colorless apolar compounds somewhat similar to alkanes but more reactive. The first few members of the series are gases or liquids at room temperature.
The simplest alkene ethylene C 2 H 4 or ethene in the IUPAC nomenclature is the organic compound. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. Butyrfentanyl N-Phenyl-N-1-2-phenylethyl-4-piperidinyl butanamide belongs to the mono-methylated fentanyl derivatives carrying the additional methyl group at the ω-carbon of the propionyl amide resulting in a butyryl amide analog of fentanyl.
Isobutyrfentanyl is the isomer carrying the additional methyl group at the α-carbon of the propionylamide moiety. Both compounds were included in. CefoTEtan cefOXitin cefTAZidime cefTRIAXone.
CeFAZolin cefOXitin cefTAZidime cefTRIAXone. CeFAZolin cefoTEtan cefTAZidime cefTRIAXone. CeFAZolin cefoTEtan cefOXitin cefTRIAXone.
CeFAZolin - cefoTEtan cefOXitin cefTAZidime. We would like to show you a description here but the site wont allow us. Experimental studies suggest that an imine obtained from the substrate amide via a radical process is the key intermediate.
Lu Synthesis 2018 50 2999-3005. Iodocyclization of unsaturated tosylamides promoted by Oxone oxidation of KI afforded in. NN-Dialkoyl-44-diaminodiphenylmethane mixtures where.
The alkoyl groups are derived from marine fatty acids C12-C24 25-Di-tert-amylhydroquinone Diamines derived from dimerized vegetable oil acids Diaryl-p-phenylenediamine where the aryl group may be phenyl tolyl or xylyl 12-Dibromo-24-dicyanobutane CAS Registry No. Furthermore S N 1 S N 2 and E1 reactions of benzylic halides show enhanced reactivity due to the adjacent aromatic ring. The possibility that these observations reflect a general benzylic activation is supported by the susceptibility of alkyl side-chains to oxidative degradation as shown in the following examples the oxidized side chain is colored.
Such oxidations are normally effected. IUPAC-nomenclatuur is een systematische manier om elementen en chemische verbindingen te benoemen zoals die door de International Union of Pure and Applied Chemistry IUPAC wordt voorgestaan. Vooral in de organische chemie wordt het systeem toegepast maar met het complexer worden van de stoffen in de anorganische chemie wordt ook daar het gebruik van systematische.
In this report a practical approach to access polysubstituted N-aryl 4-pyridones via a two-step continuous flow process under mild conditions has been developedIn comparison to batch conditions this 321 cycloaddition involving a three-components cascade reaction of aniline dialkyl ethoxymethyl enemalonate and dialkyl acetylenedicarboxylate which can provide various. Furthermore S N 1 S N 2 and E1 reactions of benzylic halides show enhanced reactivity due to the adjacent aromatic ring. The possibility that these observations reflect a general benzylic activation is supported by the susceptability of alkyl side-chains to oxidative degradation as shown in the following examples the oxidized side chain is colored.
Epoxy-activated Sepharose 6B for coupling through hydroxy amino or thiol groups via a 12-carbon spacer arm. It is particularly useful for coupling small ligands such as choline ethanolamine and sugars. The pre-activated matrix is formed by reacting Sepharose 6B with the bis oxirane 14 bis-23-epoxypropoxy-butane.
Free oxirane groups couple via stable ether bonds with hydroxyl-containing. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year.