
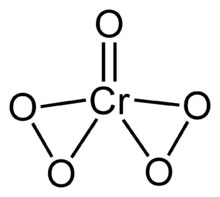
II compounds include chromiumII oxide CrO and chromiumII sulfate CrSO 4. Other chromiumVI compounds include the beautifully orange potassium dichromate K 2 Cr 2 O 7 red potassium trichromate K 2 Cr 3 O 10 and red.
Insoluble material precipitated by hydrolysis during hydrometallurgical treatment of crude zinc sulfate solution.
Chromium vi sulfate. ChromiumVI is a strong oxidising agent in contrast to the molybdenumVI and tungstenVI oxides. II compounds include chromiumII oxide CrO and chromiumII sulfate CrSO 4. Many chromiumII carboxylates are known.
The red chromiumII acetate Cr 2 O 2 CCH 3 4 is somewhat famous. It features a Cr-Cr quadruple bond. ChromiumIII Anhydrous chromiumIII chloride CrCl 3 A large.
The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr. Hexavalent chromium compounds are genotoxic carcinogensDue to its structural similarity to sulfate chromate a typical form of chromiumVI at neutral pH is transported into cells via sulfate channels. Inside the cell hexavalent chromiumVI is reduced first to pentavalent chromiumV then to trivalent chromiumIII without the aid of any enzymes.
The reduction of CrVI is considered to serve as a detoxification process when it occurs at a distance from the target site for toxic or genotoxic effect while reduction of CrVI may serve to activate chromium toxicity if it takes place in or near the cell nucleus of target organs Dayan and Paine 2001. If CrVI is reduced to CrIII extracellularly this form of the metal is not readily. Chemical compounds of chromium.
This category has only the following subcategory. Pages in category Chromium compounds The following 24 pages are in this category out of 24 total. Other chromiumVI compounds include the beautifully orange potassium dichromate K 2 Cr 2 O 7 red potassium trichromate K 2 Cr 3 O 10 and red.
Insoluble material precipitated by hydrolysis during hydrometallurgical treatment of crude zinc sulfate solution. Consists primarily of cadmium cobalt copper lead manganese nickel thallium tin and zinc. Exposure to chromium causes several toxic effects namely dermatitis allergies cancer mutations and teratogenic effects which have been attributed to hexavalent chromium ions Cr VI.
Chromates are highly toxic because they are very strong oxidizing agents and water-soluble in a wide pH range. And the structure of chromate oxyanions is like that of sulfate and phosphate ions which. Chromium is a naturally occurring element found in rocks animals plants soil and in volcanic dust and gases.
Chromium is present in the environment in several different forms. The most common forms are chromium0 chromiumIII and chromiumVINo taste or odor is associated with chromium compounds. ChromiumIII occurs naturally in the environment and is an essential nutrient.
Desulfovibrio is known as a sulfate reducing bacterium which has put it to the forefront of biological research. VI chromium VI and iron III Desulfovibrio has the potential to be both quite useful and harmful Cabrera. Desulfovibrio desulfuricans G20Image Courtesy of the Lawrence Berkeley National Laboratory.
Desulfovibrio strains have been found in a variety of. Sulfate ZnSO 4 FeSO 4 Fe 2SO 43 Ga 2SO 43 Ag 2SO 4 PbSO 42 Write the formulas for the following compounds. 1 copper II chloride CuCl 2 2 lithium acetate LiC 2H3O2 3 vanadium III selenide VSe 4 manganese IV nitride Mn 3N4 5 beryllium oxide BeO 6 sodium sulfate Na 2SO 4 7 aluminum arsenide AlAs 8 potassium permanganate KMnO 4 9 chromium VI cyanide CrCN 6 10 tin II.
Cr 2 SO 4 3. C 6 H 8 O 7. Cu 2 Cl 2.
CuNO 3 2. Sulfate sulfite thiocyanate thiosulfate ammonium hydronium C2O4 2 ClO4 IO4 MnO4 O2 2 PO4 3 P2O7 4 SO4 2 SO3 2 SCN S2O3 2 NH4 H3O POSITIVE POLYATOMIC IONS TABLE OF POLYATOMIC IONS H2PO4 HCO3 HC2O4 HSO4 HS HSO3 OH ClO IO3 HPO4 2 NO3 NO2 SiO4 4 hydrogen. ChromiumIII phosphate has the chemical formula CrPO 4.
The name of an ionic compound also gives you information about the oxidation numbers of the elements. You should know the Roman numerals for 1 I 2 II 3 III 4 IV 5 V and 6 VI. While there are higher oxidation numbers they are less common.
2 CaSO4 calcium sulfate. 3 C2Br6 dicarbon hexabromide. 4 CrCO33 chromium VI carbonate.
5 Ag3P silver phosphide. 6 IO2 iodine dioxide. 7 VO2 vanadium IV oxide.
8 PbS lead II sulfide. 10 N2O3 dinitrogen trioxide. Write the formulas of the following chemical compounds.
11 tetraphosphorus triselenide P4Se3. 58 molybdenum VI sulfate MoSO 4 3 59 platinum II sulfide PtS 60 ammonium sulfate NH 4 2 SO 4 61 NaBr sodium bromide 62 CaC 2 H 3 O 2 2 calcium acetate 63 P 2 O 5 diphosphorus pentoxide 64 TiSO 4 2 titaniumIV sulfate 65 FePO 4 iron III phosphate 66 K 3 N potassium nitride 67 SO 2 sulfur dioxide 68 CuOH copper I. With the rapid development of industrial economy heavy metals such as copper and chromium contained in industrial wastewater will cause.
The following is a list of substances NIOSH considers to be potential occupational carcinogens. A number of the carcinogen classifications deal with groups of substances. Aniline and homologs chromates dintrotoluenes arsenic and inorganic arsenic compounds beryllium and beryllium compounds cadmium compounds nickel compounds and crystalline forms of silica.
ChromiumVI compounds such as calcium chromate zinc chromates strontium chromate and lead chromates are highly toxic and carcinogenic in nature. Chromium III on the other hand is an essential nutritional supplement for animals and humans and has an important role in glucose metabolism. The uptake of hexavalent chromium compounds through the airways and digestive tract.
EPA sets legal limits on over 90 contaminants in drinking water. The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology. Généralité sur lélément et le corps simple histoire et lexique.
Le chrome appartenant au groupe 6 et à la période 4 du tableau périodique fait partie de la famille des métaux de transition. Il fait partie du sixième groupe secondaire du tableau périodique en un sens restreint du groupe chimique du chrome ou groupe VI B qui comporte également le molybdène Mo et le tungstène W 8. 56 Chromium VI It is recommended that valence-specific data for chromium be collected whenever possible when chromium is likely to be an important contaminant at a site and when hexavalent chromium Cr VI may exist.
For CrVI IRIS shows an air inhalation unit risk IUR of 12E-2 per µgm 3. While the exact ratio of CrVI to CrIII. Increases in serum sulfate may explain some of the therapeutic effects of MSM DMSO and glucosamine sulfate.
Organic sulfur as SAAs can be used to increase synthesis of S-adenosylmethionine SAMe glutathione GSH taurine and N-acetylcysteine NAC. MSM may be effective for the treatment of allergy pain syndromes athletic injuries and bladder disorders. Other sulfur compounds such as.
Sterile outlet connection diam. 916 in pore size 02 μm pore size 05 μm cartridge nominal length 47 in. How the EPA conducts risk assessment to protect human health and the environment.
Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments.