Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. FDA Center for Food Safety and Applied Nutrition CFSAN FDA Color Additive Status for.
However it is still used in data tape applications for enterprise-class storage systems.
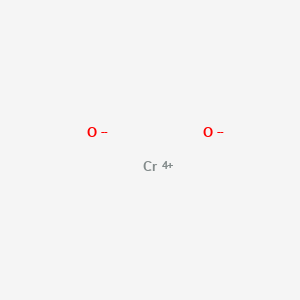
Chromium iv oxide. Chromium dioxide or chromiumIV oxide is an inorganic compound with the formula CrO 2. It is a black synthetic magnetic solid. It once was widely used in magnetic tape emulsion.
With the increasing popularity of CDs and DVDs the use of chromiumIV oxide has declined. However it is still used in data tape applications for enterprise-class storage systems. It is still considered by many.
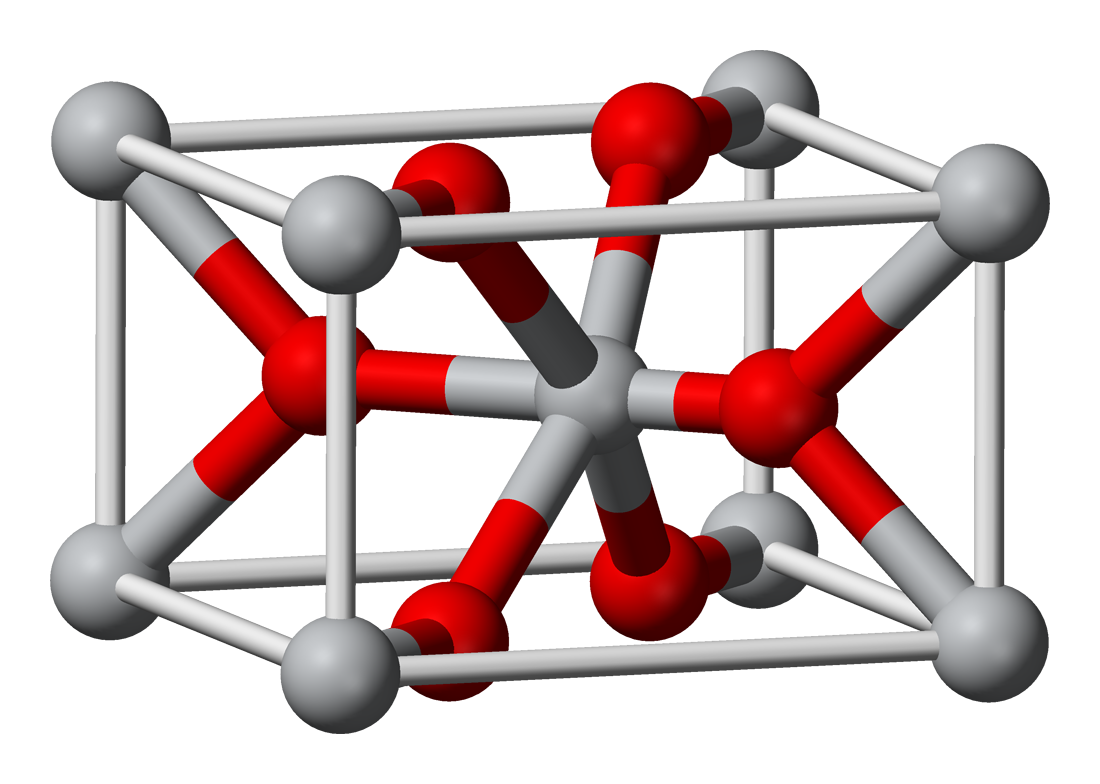
ChromiumIII oxide or chromia is an inorganic compound with the formula Cr 2 O 3. It is one of the principal oxides of chromium and is used as a pigment. In nature it occurs as the rare mineral eskolaite Structure and properties.
Cr 2 O 3 has the corundum structure consisting of a hexagonal close packed array of oxide anions with 2 3 of the octahedral holes occupied by chromium. Chromium oxide CrO2 chromiumIV oxide. Medical Subject Headings MeSH 242 Depositor-Supplied Synonyms.
Chromium oxide Cr3O4 Chromium Oxide Nanoparticles Nanopowder. Chromium oxide Cr2O5 Chromic acid chromium salt. Chromium oxide greens - Color additives exempt from certification and permanently listed for COSMETIC use.
None of these colors may be used in products that are for use in the area of the eye unless otherwise indicated. Externally applied cosmetics including those for eye area - GMP - 732327. FDA Center for Food Safety and Applied Nutrition CFSAN FDA Color Additive Status for.
Lead IV oxide. Naming Covalent Compounds Naming B inary Ionic Compounds Polyatomic Ions Naming with Polyatomic Ions. 4 manganese IV nitride Mn 3N4 5 beryllium oxide BeO 6 sodium sulfate Na 2SO 4 7 aluminum arsenide AlAs 8 potassium permanganate KMnO 4 9 chromium VI cyanide CrCN 6 10 tin II sulfite SnSO 3 11 vanadium V fluoride VF 5 12 ammonium nitrate NH 4NO 3.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N.