Cs cesium Zn 2. When heated with finely divided carbon or aluminium it is reduced to chromium metal.
The primary aim of the cards is to promote the safe use of chemicals in the workplace.
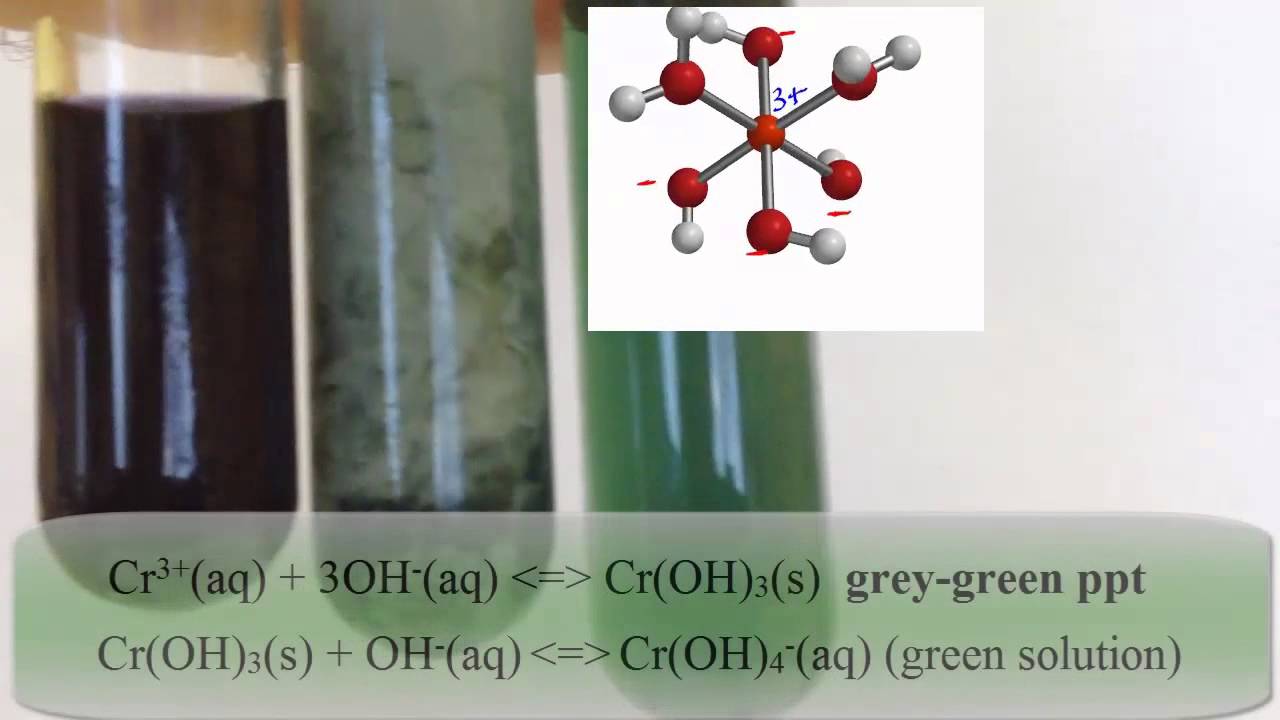
Chromium iii peroxide. ChromiumIII oxide is amphoteric. Although insoluble in water it reacts with acid to produce salts of hydrated chromium ions such as CrH 2 O 6 3. It is also attacked by concentrated alkali to yield salts of CrOH 6 3.
When heated with finely divided carbon or aluminium it is reduced to chromium metal. Cr 2 O 3 2 Al 2 Cr Al 2 O 3. Unlike the classic thermite reaction.
ChromiumIII can be obtained by dissolving elemental chromium in acids like hydrochloric acid or sulfuric acid. The unstable dark blue chromiumVI peroxide CrO 5 is formed which can be stabilized as an ether adduct CrO 5 OR 2. Chromic acid has the hypothetical formula H 2 CrO 4.
It is a vaguely described chemical despite many well-defined chromates and dichromates being known. Metallic chromium dissolves in dilute hydrochloric acid forming CrII and hydrogen gas H 2In aqueous solution CrII is present as the complex ion CrOH 2 6 2Similar results are seen for sulphuric acid but pure samples of chromium may be resistant to attack. In an industrial hygiene survey of 60 ferrochromium workers exposed to CrIII and CrVI 002-019 mg total chromiumm³ conducted in 1975 appreciably higher incidences of subjective symptoms of coughing wheezing and dyspnea were reported compared with controls.
However due to the tobacco smoking that cannot be excluded as a confounding factor the increase in subjective respiratory. List of Common Ions Polyatomic Cations NH4 ammonium H3O hydronium Polyatomic Anions OH-hydroxide CN-cyanide O2 2-peroxide CO3 2-carbonate C2O4 2-oxalate NO2-nitrite NO3-nitrate PO3 3-phosphite PO4 3-phosphate SO3 2-sulfite SO4 2-sulfate S2O3 2-thiosulfate ClO-hypochlorite ClO2-chlorite ClO3-chlorate ClO4-perchlorate CH3COO or C2H3O2-acetate AsO4 3-arsenate. CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice.
Complete these in lab and on your own time for practice. Potassium peroxide K 2O 2. II sulfate CrSO 4 chromium III hydrogen sulfate CrHSO 4 3 iron III acetate FeCH 3COO 3 silver peroxide Ag 2O 2 tin IV iodite SnIO 2 4 lead IV hydrogen chromate PbHCrO 4 4 lithium peroxide Li 2O 2 cobalt II perchlorate CoC ℓO 4 2 arsenic V thiosulfate As 2S 2O 3 5 gold III fluoride AuF 3 calcium permanganate CaMnO 4 2 sodium peroxide.
Ferrous ion ironii is oxidized to ferric ion ironiii and H 2 O 2 is reduced to water. Green colour of Fe 2 solution is changed to brown - yellow colour due to production of Fe 3. 2FeCl 2 H 2 O 2 3H 2 SO 4 Fe 2 SO 4 3 4HCl 2H 2 O.
Sulfurous acid and Hydrogen Peroxide Reaction H 2 SO 3 H 2 O 2 H 2 SO 4 H 2 O. Sulfurous acid is oxidized to sulfuric acid. Titanium III V3 vanadiumIII V5 vanadium V Cr3 Cr2 chromium III chromium II Mn2 Mn4 manganeseII manganeseIV Fe3 Fe2 iron III iron II Co2 Co3 cobalt II cobalt III Ni2 Ni3 nickel II nickel III Cu2 Cu copper II copper I Ga3 gallium Sc3 scandium Y3 yttrium La3 lanthanum Ac3 actinium Zr4 zirconium Hf4.
Students enrolled in Dr. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below. Chromium 52Cr 00220 00546 00539 0489 539 489.
UltraWAVE and UltraCLAVE III systems. 3 LabwareAll laboratory ware must be sufficiently clean for trace metals analysis. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. 4-2 sulfate SO 3-2 sul fite.
Cations 1 Cations 2 Cations 3 Cations. H hydrogen Be2 beryllium Al3 aluminum Li lithium Mg2 magnesium. Na sodium Ca2 calcium K potassium Sr2 strontium.
Rb rubidium Ba 2. Cs cesium Zn 2. Ag silver Cd 2.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. ChromiumIII forms a steel green hydroxide which dissolves in excess strong base to give a deeply green colored solution of the hydroxy complex. Treating this complex with 3 hydrogen peroxide gives the yellow solution of the chromate ion which upon acidification with dilute nitric acid gives the orange color of dichromate.
Treatment of the cold solution of dichromate with 3 hydrogen.