ChromiumIII phosphate has the chemical formula CrPO 4. Naming Ionic Compounds Practice Worksheet - Solutions Name the following ionic compounds.

It is one of the principal oxides of chromium and is used as a pigment.
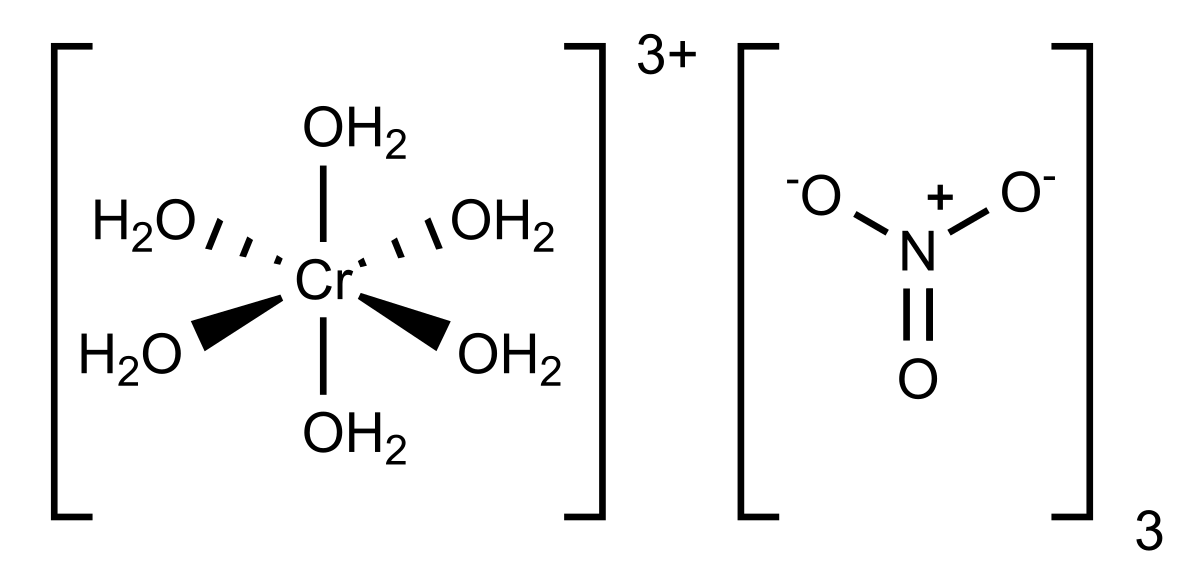
Chromium iii nitrate. ChromiumIII oxide or chromia is an inorganic compound with the formula Cr 2 O 3. It is one of the principal oxides of chromium and is used as a pigment. In nature it occurs as the rare mineral eskolaite.
Cr 2 O 3 has the corundum structure consisting of a hexagonal close packed array of oxide anions with 2 3 of the octahedral holes occupied by chromium. When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII hydroxide CrOH3 forms. Write a balanced net ionic equation for this.
Aqueous potassium phosphate was mixed with aqueous magnesium chloride and a crystallized magnesium phosphate product was formed. CID 944 Nitric acid CID 23976 Chromium Dates. Chromium trinitrate is an inorganic nitrate salt consisting of chromium and nitrate in which the ratio of chromium in the 3 oxidation state to nitrate is 13 It is.
Vitamin C and other reducing agents combine with chromate to give chromiumIII products inside the cell. The resultant chromiumIII. Hexavalent chromium was released from the Newcastle Orica Koorgang Island ammonium nitrate plant on August 8 2011.
The incident occurred when the plant entered the start up phase after the completion of a five-yearly maintenance overhaul. Chromium is a naturally occurring element found in rocks animals plants soil and in volcanic dust and gases. Chromium is present in the environment in several different forms.
The most common forms are chromium0 chromiumIII and chromiumVINo taste or odor is associated with chromium compounds. ChromiumIII occurs naturally in the environment and is an essential nutrient. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Since lead has more than one oxidation state we must figure out which lead we have. Since each nitrate 4 of them has a 1- charge the Pb must be 4.
So our roman numeral will be IV. PbNO 3 4 is named leadIV nitrate Highlight to reveal names. Cobalt III bromide.
Iron III phosphide. IronIII V and IV Jones Reagent. Meta-Chloroperbenzoic acid Methoxycarbonylsulfamoyltriethylammonium hydroxide.
MMPP 6 H 2 O. Chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III cobaltic Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin. 3 vanadium III selenide VSe 4 manganese IV nitride Mn 3N4 5 beryllium oxide BeO 6 sodium sulfate Na 2SO 4 7 aluminum arsenide AlAs 8 potassium permanganate KMnO 4 9 chromium VI cyanide CrCN 6 10 tin II sulfite SnSO 3 11 vanadium V fluoride VF 5 12 ammonium nitrate NH 4NO 3.
ChromiumIII nitrate aq ironII sulfate aq chromiumIII sulfate aq ironII nitrate aq Molecular. 2 CrNO 3 3 aq 3 FeSO 4 aq 3 FeNO 3 2 aq Cr 2 SO 4 3 aq. Fe3 Fe2 iron III iron II atomic 26 number ion charge ion name symbol IUPAC KEY 58 59 60 61 62 63 64 65 66 67 68 69 70 71.
Potassium nitrate may decrease the excretion rate of Chromium nicotinate which could result in a higher serum level. Potassium nitrate may decrease the excretion rate of Chromous sulfate which could result in a higher serum level. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
This is our newest publication and has been created to support the school technician profession in Scotland. A Write a balanced equation for the reactants given. B Include the physical states for all reagents.
Assume that all reactions are in water. Nitrate Method 8192 Cadmium Reduction Method Powder Pillows LR 001 to 050 mgL NO3 N Nitrate Method 10020 Chromotropic Acid Method Test N Tube Vials HR 02 to 300 mgL NO3N Nitrate Method 10206 Dimethylphenol Method TNTplus 836 HR 535 mgL NO3N or 22155 mgL NO3 Nitrate Method 10206 Dimethylphenol Method TNTplus 835 LR 023. ChromiumIII phosphate has the chemical formula CrPO 4.
The name of an ionic compound also gives you information about the oxidation numbers of the elements. You should know the Roman numerals for 1 I 2 II 3 III 4 IV 5 V and 6 VI. While there are higher oxidation numbers they are less common.
The restricted substances on their own in a mixture or in an article are substances for which manufacture placing on the market or use is limited or banned in the European Union. Naming Ionic Compounds Practice Worksheet - Solutions Name the following ionic compounds. 1 NH4Cl ammonium chloride 2 FeNO33 iron III nitrate 3 TiBr3 titanium III bromide 4 Cu3P copper I phosphide 5 SnSe2 tin IV selenide 6 GaAs gallium arsenide.
The SEMS Search allows you to retrieve Superfund data from the Superfund Enterprise Management System SEMS database in Envirofacts. Specify a facility by using any combination of facility name and geographic location. 41 Restrictions Annexes III IV Directive 201165EU RoHS as amended by Directive EU 2021884 L 194 2 June 2021.
This list contains the application exemptions listed in Annexes III and IV to RoHS 201165EU. Annex III contains applications exempted from the restriction in Article 41 while Annex IV lists applications exempted from the restriction in. Therefore the oxidation number of chromium must be the same as the charge of the complex ion 3.
PtNH 3 5ClBr 3 Answer. The complex ion is a cation and the counter anions are the 3 bromides. The charge of the complex ion must be 3 since it is associated with 3 bromides.
The NH 3 molecules are neutral while the chloride. Chemical or scientific names are used to give an accurate description of a substances composition. Even so you rarely ask someone to pass the sodium chloride at the dinner table.
Its important to remember that common names are inaccurate and vary from one place and time to another. Desulfovibrio cells typically grow anaerobically but certain strains have been identified growing in the presence of oxygenSpecies of Desulfovibrio are traditionally Gram-negative non-spore forming and about 07 um in cell diameterDesulfovibrio is known for its flexbility in response to the extended amount of electron acceptors it utilizes including sulfate sulfur nitrate and nitrite. The catalase activity stimulated with excess supply of chromium inducing toxicity has been studied with respect to photosynthesis nitrate reductase activity protein content in algae and photosynthetic pigments Nath et al 2008.
Chromium III requires a simple diffusion process to enter into the cell and does not depend on any specific membrane carrier. In contrast to CrIII CrIV can.