
Ionic Compounds with Polyatomic Ions. Ammonium chlorate NH4ClO3 78.
It is amphoteric dissolving in both strong alkalis and strong acids.
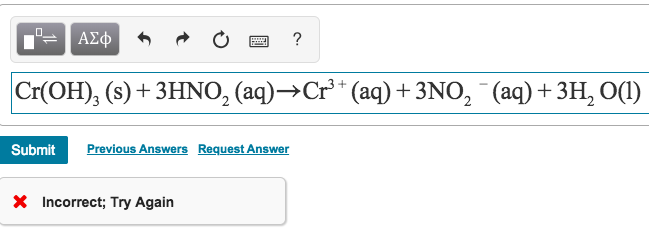
Chromium iii hydroxide formula. ChromiumIII hydroxide is a gelatinous green inorganic compound with the chemical formula CrOH 3. It is a polymer with an undefined structure and low solubility. It is amphoteric dissolving in both strong alkalis and strong acids.
CrOH 3 OH CrO 2 2 H 2 O In acid. CrOH 3 OH 2 3 3 H CrOH 2 3 6. It is used as a pigment as a mordant and as a.
ChromiumIII hydroxide CrOH 3 is amphoteric dissolving in acidic solutions to form Cr H 2 O 6 3 and in basic solutions to form CrOH 6 3. It is dehydrated by heating to form the green chromiumIII oxide Cr 2 O 3 a stable oxide with a crystal structure identical to that of corundum. ChromiumVI ChromiumVI compounds are oxidants at low or neutral pH.
Chromate anions CrO. The oxidation of chromiumIII to chromiumVI An excess of sodium hydroxide solution is added to a solution of the hexaaquachromiumIII ions to produce a solution of green hexahydroxochromateIII ions. This is then oxidised by warming it with hydrogen peroxide solution.
You eventually get a bright yellow solution containing chromateVI ions. The intestinal absorption of trivalent and hexavalent chromium Cr given orally experiment I or infused in the intestine experiment II was investigated in rats. The nonabsorbable form of chromium 51Cr2O3 and water-soluble and more absorbable Na251CrO4 the hexavalent form of Cr were comparedTotal retention of chromium given orally ranged around 15 percent of the dose regardless.
The ionic compound without the waters of hydration is named first by using the rules for naming ionic compounds eg BaOH 2 8H 2 O barium hydroxide. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. 8 water molecules octa hydrate.
Name Formula Charge Name Formula Charge Name Formula Charge. Chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III cobaltic Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV. 2 NaOH sodium hydroxide 3 MgBr 2 magnesium bromide 4 KCl potassium chloride 5 FeCl 2 iron II chloride 6 FeCl 3 iron III chloride 7 ZnOH 2 zinc hydroxide 8 Be 2 SO 4 beryllium sulfate 9 CrF 2 chromium II fluoride 10 Al 2 S 3 aluminum sulfide 11 PbO lead II oxide 12 Li 3 PO.
The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr. Ionic Compound Formula K sp. Aluminum hydroxide AlOH 3 1810 5.
Aluminum phosphate AlPO 4 6310 19. Barium carbonate BaCO 3 5110 9. Barium chromate BaCrO 4 1210 10.
Barium fluoride BaF 2 1010 6. Barium hydroxide BaOH 2 510 3. Barium sulfate BaSO 4 1110 10.
Barium sulfite BaSO 3 810 7. Barium thiosulfate BaS 2 O 3 1610 6. Chemical formula plays an important role in understanding different concepts of chemistry.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
Calcium hydroxide 98 FeOH 3 iron III hydroxide Write the formulas of the following acids and bases. 99 hydrobromic acid HBr 100hydrofluoric acid HF 101 carbonic acid H 2 CO 3 102 lithium hydroxide LiOH 103 nitrous acid HNO 2 104 cobalt II hydroxide CoOH 2 105 sulfuric acid H 2 SO 4 106 beryllium hydroxide BeOH 2. Chromium III sulfate Cr2SO43 77.
Zinc hydroxide ZnOH2 67. Ammonium chlorate NH4ClO3 78. Sodium carbonate Na2CO3 68.
Mercury II chromate HgCrO4 79. Lead IV oxide PbO2 69. Silver phosphate Ag3PO4 80.
Students enrolled in Dr. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below. Ionic Naming Practice Problems - Solutions Name the following ionic compounds.
1 NaBr sodium bromide 2 ScOH3 scandium hydroxide 3 V2SO43 vanadium III sulfate 4 NH4F ammonium fluoride 5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. 3 IronIII hydroxide Cr 3PO 4 2 ChromiumII phosphate CrPO 4 ChromiumIII phosphate NaHCO 3 Sodium hydrogen carbonate or sodium bicarbonate 3.
Acids and Acid Salts. Acids are compounds in which the cation is H. These are not really ionic compounds but well get into that later These can be named as compounds as in the previous cases eg H Cl is hydrogen chloride but.
Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound.
ChromiumIII oxide _ Cr2O3_ tinII fluoride __ SnF2__ ironIII iodide _ FeI3__ zinc nitride _ Zn3N2__ copperII bromide __ CuBr2_ cobaltII oxide __ CoO__ This information is only included so that if you see these names elsewhere youll have some idea why. You do not need to learn these for my class. Ionic Compounds with Polyatomic Ions.
Cases 1 and 2 above involve ionic. The name of a cationic complex ion ends in the name of the central metal ion with the oxidation state shown as a Roman numeral in parantheses at the end of the metals name eg ironIII. 5 The name of an anionic complex ion ends in ate chromiumII chromateII nickelII nickelateII.
How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Based on the chemical formula of a substance we know the composition of the substance.