Copper I Cyanide SDS. Liquid substances or mixtures which are regarded as dangerous in accordance with Directive 199945EC or are fulfilling the criteria for any of the following hazard classes or categories set out in Annex I to Regulation EC No 12722008 See group members.

In the solid these water molecules also called waters of hydration are part of the structure of the compound.
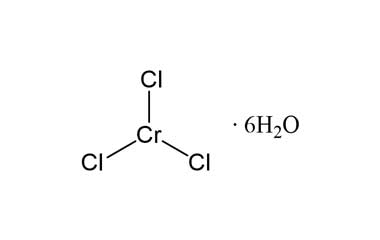
Chromium iii chloride hexahydrate. ChromiumIII chloride also called chromic chloride describes any of several compounds of with the formula CrCl 3 x H 2 O where x can be 0 5 and 6. The anhydrous compound with the formula CrCl 3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate CrCl 3 6H 2 OChromium chlorides find use as catalysts and as precursors to dyes for wool.
Hydrated ionic compounds ie hydrates have a specfic number of water molecules in their chemical formulas. In the solid these water molecules also called waters of hydration are part of the structure of the compound. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass.
These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. During my early chemical investigations I acquired some chromiumIII chloride hexahydrate a green salt that gave an equally green solution when dissolved in water. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. IronIII V and IV Jones Reagent. Meta-Chloroperbenzoic acid Methoxycarbonylsulfamoyltriethylammonium hydroxide.
MMPP 6 H 2 O. Write the formula for each compound. Systematic name Asked for.
A Identify the cation and its charge using the location of the element in the periodic table and Table 22 Some Common Monatomic Ions and Their Names Table 23 The Physical Properties of Typical Ionic Compounds and Covalent. Liquid substances or mixtures which are regarded as dangerous in accordance with Directive 199945EC or are fulfilling the criteria for any of the following hazard classes or categories set out in Annex I to Regulation EC No 12722008 See group members. Showhide Hazard class 41 EC No.
- CAS No. - Hazard classes 31 to 36 37 adverse. Citric Acid 10 SDS.
Citric Acid 25 SDS. Citric Acid Anhydrous SDS. Coal Tar Solution SDS.
Cobalt Sulfate Crystal Reagent Grade. Copper I Cyanide SDS. Cupric Nitrate 10 Molar.
Cupric Sulfate Pentahydrate SDS. Chromiumiii nitrate nonahydrate icsc chromiumiii oxide icsc chromiumiii chloride hexahydrate icsc tert-butyl chromate icsc strontium icsc tin icsc sodium benzoate icsc hydroxyacetic acid icsc aluminium phosphate tribasic icsc undecyl alcohol icsc pigment red 53 barium salt 21 icsc 2-nitro-p-phenylenediamine icsc. USA government specifications for chromium trioxide are as follows.
Water insoluble materials 010 maximum. Particle size 10 minimum passes through -30 mesh less than 590 um US sieve. Chromium chloride 6 h2o.
Chromium3 chloride hydrate 136 氯化铬 物理性质 编辑 语音. For example cobaltII chloride hexahydrate only loses four water molecules to become cobaltII chloride dihydrate when heated in an oven at 150 C. Suggest an explanation for this observation.
Although the chemical formula might suggest that waters in transition metal compounds are hydrates often some of the waters act as ligands that chemically bond in some cases fairly strongly to the. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
Chromium III Insoluble Salts 16065-83-1. By the MW of lanthanum chloride anhydrous 24527. To derive the chronic oral RfD of 187E-05 mgkg-day for lanthanum chloride heptahydrate the lanthanum RfD of 5E-05 mgkg-day is multiplied by the MW of lanthanum 13891 divided by the MW of lanthanum chloride heptahydrate 37137.
To derive the chronic oral RfD of. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. M m A n s mM n aq nA m-aq.
Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. K sp M n m A m- n. The table below gives calculated values of K.
Chromium III chloride and its hexahydrate. Chromium III sulphate and its hexahydrate. Ammonium molybdate molybdenum VI sodium molybdate molybdenum VI ANNEX III.
SUBSTANCES WHOSE USE IN FOODS IS PROHIBITED RESTRICTED OR UNDER COMMUNITY SCRUTINY. Part A Prohibited substances. Part B Restricted substances.
Part C Substances under. How the EPA conducts risk assessment to protect human health and the environment. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments.
CobaltIIchloride Hexahydrate Pink crystalline solidsolution icolour changes from pink to blue. Ii colourless liquid forms on the cooler parts of boiling test tube if crystal are used icolour changes from blue to pink iiboiling tube becomes warmhot. When blue CopperIISulphate VI pentahydrate is heated it loses the five molecules of water of crystallization to form white.
Academiaedu is a platform for academics to share research papers.