
25 Di Chloro Nitro Benzene. The simplified formula for this is C 6 H 5 NO 2.
IC50 of 23 nM.
Chloro nitro benzene. 25 Di Chloro Nitro Benzene. Para Nitro Aniline. 34 Di Chloro Nitro Benzene.
34 Di Chloro Aniline. Sulphuric Acid Group Product. The conversions of the 4-nitro derivative are similar.
Chlorobenzene once was used in the manufacture of certain pesticides. Chlorobenzene is manufactured by chlorination of benzene in the presence of a catalytic amount of Lewis acid such as ferric chloride sulfur dichloride and anhydrous aluminium chloride. The catalyst enhances the electrophilicity of the chlorine.
Because chlorine is. Halogen and nitro substituents on an aromatic ring even remain unaffected by these oxidations. For example chromic acid oxidizes 2-chloro-4-nitrotoluene to produce 2-chloro-4-nitrobenzoic acid.
But here as well the nitro and chloro group remain unaffected. Only those codes applicable to the University of Maryland are listed Hazardous waste is any solid waste that either exhibits any of the characteristics of hazardous waste or is a listed EPA waste. In addition EPA Hazardous Waste Codes are also classified as acute and non-acute.
P-listed codes and certain dioxin codes F020-F023 and F026-F028 are considered to be acute. How the EPA conducts risk assessment to protect human health and the environment. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments.
A prefix appearing in loanwords from Greek with the meanings at or to one side of beside side by side parabola. Paragraph beyond past by paradox. By extension designating objects or activities auxiliary to or derivative of that denoted by the base word parody.
Paronomasia and hence abnormal or defective paranoiaAs an English prefix para-1 is also productive in. The nitro group NO 2 is attached to a benzene ring. The simplified formula for this is C 6 H 5 NO 2.
Another obvious name - the benzene ring has a methyl group attached. Other alkyl side-chains would be named similarly - for example ethylbenzene. The old name for methylbenzene is toluene and you may still meet that.
The simplified formula for this is C 6 H 5 CH 3. For instance in a nitration protocol 4-chloro-n-butylbenzene is reacted with sodium nitrite in t-butanol. This is done in the presence of 05 mol Pd 2 dba 3 a biaryl phosphine ligand and a phase-transfer catalyst PTC to form 4-nitro-n-butylbenzene.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health.
The ICSC project is a common undertaking between the World Health Organization WHO and. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Stearoyl-CoA Desaturase 1 Inhibitor MF-438 CAS 921605-87-0 is a cell-permeable inhibitor of Stearoyl-CoA Desaturase 1 SCD1.
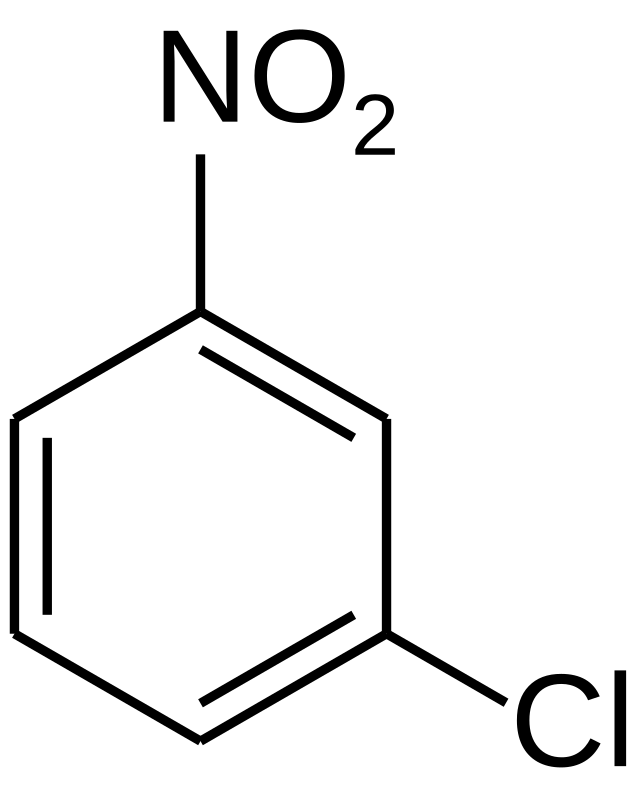
IC50 of 23 nM. For example chlorine Cl attached to a phenyl group would be named chlorobenzene chloro benzene. Since there is only one substituent on the benzene ring we do not have to indicate its position on the benzene ring as it can freely rotate around and you would end up getting the same compound Figure 8.
Example of simple benzene naming with chlorine and NO 2 as substituents. Erno Pretsch Philippe Buhlmann Martin Badertscher Structure Determination of Organic Compounds Tables of Spectral Data Fourth Revised and Enlarged Edition 123. Electrophilic Substitution of Disubstituted Benzene Rings.
When a benzene ring has two substituent groups each exerts an influence on subsequent substitution reactions. The activation or deactivation of the ring can be predicted more or less by the sum of the individual effects of these substituents. The site at which a new substituent is introduced depends on the orientation of the existing.
Piperonyl Methyl Ketone PMK 112-55-0. 1-Dodecanethiol n-Dodecyl mercaptan NDM. Enter the AG Number for the specific 60-day Notice for which you are filing a report.
2018-00010 If you do not have the AG Number search the database to find the AG Number and select the specific 60-day Notice you need. Toxicity criteria on chemicals evaluated by OEHHA. OEHHA chemical database meta data Export database as CSV file If you are having trouble with the download and would like a copy of the database just drop me LaurieMonserratoehhacagov a note and I will provide you a csv file.
If only inhalation and oral exposure occurs the benzene MADL is exceeded when. Oral dose 24 μgday inhalation dose 49 μgday 10. B Levels for male children and adolescents were calculated by application of the default bodyweights specified in Section 25703a8 to the procedure specified in Sections 25801 and 25803 Title 27 California Code of Regulations.
In organic chemistry the phenyl group or phenyl ring is a cyclic group of atoms with the formula C 6 H 5Phenyl groups are closely related to benzene and can be viewed as a benzene ring minus a hydrogen which may be replaced by some other element or compound to serve as a functional groupPhenyl groups have six carbon atoms bonded together in a hexagonal planar ring five of which are. Acetic acid fluoro- sodium salt. 1 4 Benzodioxane carbonyl piperazine HCLBase.
9-Methyl-1239- Tetrahydro-4-H Carbazole 4-one. 1-2 4-Dichloro phenyl-2-1H-Imidazol-yl Ethanol. How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes.
Last time we covered a comparatively simple reaction. Free-radical chlorination of methane to CH 4 to give chloromethane CH 3 Cl and saw that the reaction proceeds through three stages initiation where free radicals are created propagation the main product-forming step of the chain. Surface tension 20 C in mNm.
Temperature coefficient in mNm K 12-Dichloro ethane. A1-cbiss sell gas detection equipment and continuous emissions monitoring systems. The core of a1-cbiss success is built with a strong emphasis on engineering design and technical experience supported by the industrys largest service network in the UK.
Both chloro and the nitro groups deactivate the amine group to n-hydroxylation and the ring to epoxidation no active products from cytochrome p450 would be predicted. The activity of the nitro derivatives is presumed to be due to transformation of the nitro group itself to an active mutagenic species by other enzyme systems.