Write the name or formula of each compound or element in the following paragraph. Contact with organic materials may result in spontaneous ignition.
It reacts with water to form chlorine and hydrofluoric acid with release of heat.
Chlorine trifluoride formula. Chlorine trifluoride is an interhalogen compound with the formula ClF 3. This colorless poisonous corrosive and extremely reactive gas condenses to a pale-greenish yellow liquid the form in which it is most often sold pressurized at room temperature. The compound is primarily of interest as a component in rocket fuels in plasmaless cleaning and etching operations in the semiconductor.
Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. It reacts with water to form chlorine and hydrofluoric acid with release of heat. Contact with organic materials may result in spontaneous ignition.
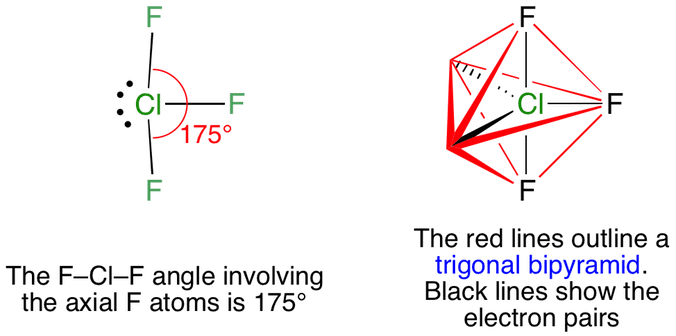
What is the Hybridization of Chlorine Trifluoride. When we talk about the hybridization of chlorine trifluoride we have to consider its central atom which is Cl. This atom contains 7 valence electrons while ClF3 should consist of 3 bond-pairs and 2 lone-pairs.
If we take a closer. Chlorine Trifluoride ClF 3 Chlorine Trifluoride is a strong oxidizer agent known as the most flammable substances because it doesnt even need ignition to start a fire even initiate the combustion of many non-flammable materials without any ignition source. This colorless poisonous corrosive and reactive gas is commonly used for military purpose rocket fuels plasmaless cleaning.
Molecular geometry of ClF3. Electron geometry of ClF3. Total Valence electron in ClF3 lewis structure.
The formal charge of ClF3. How to draw ClF3 lewis structure. ClF3 lewis structure contains 3 fluorine atoms at the surrounding position and 1 chlorine.
For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine. The element with the lower group number is written first in the name. The element with the higher group number is written second in the name.
When the compound contains oxygen and a halogen the name of the halogen is the first word in the name. Phosphorus trifluoride formula P F 3 is a colorless and odorless gas. It is highly toxic and reacts slowly with water.
Its main use is as a ligand in metal complexes. As a ligand it parallels carbon monoxide in metal carbonyls and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide. Phosphorus trifluoride has.
Chlorine dioxide hydrogen iodide iodine pentafluoride dinitrogen trioxide phosphorus triiodide Phosphorus trihydride Dichlorine heptoxide Boron trifluoride Silicon dioxide Silicon tetrafluoride Carbon monoxide Dihydrogen monoxide Dihydrogen dioxide Nitrogen trihydride Dicarbon dihydride Dinitrogen tetrahydride Sulfur trioxide Dichlorine monoxide Carbon tetrahydride Dinitrogen monoxide Sulfur. We present an analytical method for the in situ measurement of atmospheric nitrogen trifluoride NF3 an anthropogenic gas with a 100-year global warming potential of over 16000. This potent greenhouse gas has a rising atmospheric abundance due to its emission from a growing number of manufacturing processes and an expanding end-use market.
Here we present a modified version of the Medusa. Acceleration Formula Force Formula Frequency Formula Velocity Formula Wavelength Formula Angular Velocity Formula Displacement Formula Density Formula Kinematic Equations Formula Tangential Velocity Formula Kinetic Energy Formula Angular Speed Formula Buoyancy Formula Efficiency Formula Static Friction Formula Potential Energy. Chlorine Cl2 ammonia NH3 methane CH4 and carbon monoxide CO Exercise.
Write the name or formula of each compound or element in the following paragraph. Probably the most important element found uncombined in nature is O2. O2 is quite reactive forming compounds with the halogens F2 Cl2 Br2 and I2.
O2 forms two compounds with H2. Let us practice by naming the compound whose molecular formula is CCl 4The name begins with the name of the first elementcarbon. The second element chlorine becomes chloride and we attach the correct numerical prefix tetra- to indicate that the molecule contains four chlorine atomsPutting these pieces together gives the name carbon tetrachloride for this compound.
Sulfuric acid 98072 g. GAS NAME FORMULA COLUMN A COLUMN B Acetylene Ethyne C2H2 137 437 Air 08N2-02O2 122 889 Allene C3H4 88 _____ Ammonia NH3 209 1188 Argon Ar 89 1035 Arsine AsH3 46 _____ Boron Trichloride BCl3 31 _____ Boron Trifluoride BF3 52 707 13-Butadiene C4H6 63 _____ Butane C4H10 61 298 1-Butene C4H8 62 _____ 2-Butene C4H8 62 _____ Carbon Dioxide CO2 81 684 Carbon Monoxide. Trifluoride Bromine Water Calcium Carbonate Chloride Hydroxide Nitrate Sulfate Carbon Dioxide Disulfide Tetrachloride Chlorine Atomic Chlorine Chlorine Dioxide Chlorosulfonic Acid Chromium Trioxide Copper Chloride ll Nitrate ll Sulfate ll Solid Dyneon PVDF Temperature Of Medium C F Inorganic Media 2 228 239 212 212 224.
A chlorine atom Group VIIA has seven valence electrons and each oxygen atom Group VIA has six valence electrons. Because the chlorate ion has a charge of -1 this ion contains one more electron than a neutral ClO 3 molecule. Thus the ClO 3-ion has a total of 26 valence electrons.
7 36 1 26. The second step in this process involves deciding which atoms in the molecule are. 1 ppm 115 mgm 3.
NIOSH REL TWA 35 ppm 40 mgm 3 C 200 ppm 229 mgm 3 OSHA PEL TWA 50 ppm 55 mgm 3 See Appendix G. OSHA ID209 ID210 See. NMAM or OSHA Methods.
Shipped as a nonliquefied or liquefied. Learn the hazard class of toxic and hazardous gases. Find everything you wanted to know about compressed gases including hazard class description and hazards Hazard Control Plan regulatory information signs and symptoms of exposure and more on the Toxic and Hazardous Gas Classifications Chart below or download the entire Toxic and Hazardous Gas Classifications Chart PDF.
Formula Reaction conditions Description Acidic Buffer. Chlorine Trifluoride 3 parts Fluorine. 1 part Chlorine Temperature 424K When created it will create a temporary 3x3 fireball.
Becomes 1000K when first mixed. A flammable substance so dangerous it can instantly melt floor or floor plating with high amounts. It lights you on fire when it comes into contact with you and if it gets inside.
Phosphorus pentachloride PCl 5 sulfur hexafluoride SF 6 chlorine trifluoride ClF 3 and the triiodide ion I 3 are examples of hypervalent molecules. For the elements in the second period of the periodic table principal energy level n2 the s 2 p 6 electrons comprise the octet and no d sublevel exists. Making a carbon-chlorine double bond would satisfy the octet rule but.
Boron trifluoride 24 valence electrons 3 3x7 The octet rule is not satisfied on the B but the formal charges are all zero. In fact trying to make a boron-fluorine double bond would put a positive formal charge on fluorine. Since fluorine is highly electronegative this is extremely unfavorable 15.
In the nuclear industry chlorine trifluoride is used to prepare uranium hexafluoride a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction latextextCl_2g3textF_2glongrightarrow2textClF_3glatex. Write the equation that relates the rate expressions for this reaction in terms of the.