This study demonstrates the viability of the thiolatedisulfide redox couple in AZIB applications and provides an in-depth study on the electrochemical mechanism of Zn-thiolates electrode materials. Digestion of food 48.
Digestion of food 48.
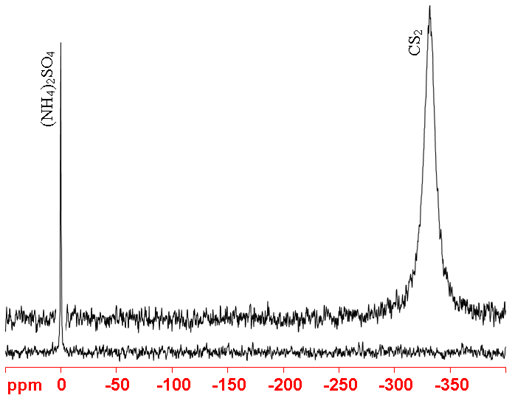
Chemical shift of carbon disulfide. Carbon dioxide chemical formula CO 2 is an acidic colorless gas with a density about 53 higher than that of dry air. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earths atmosphere as a trace gasThe current concentration is about 004 412 ppm by volume having risen from pre-industrial levels of 280 ppm.
Thioldisulfide exchange is a chemical reaction in which a thiolate group S attacks a sulfur atom of a disulfide bond SS. The original disulfide bond is broken and its other sulfur atom is released as a new thiolate carrying away the negative charge. Meanwhile a new disulfide bond forms between the attacking thiolate and the original sulfur atom.
Carbon tetrachloride is a manufactured chemical that does not occur naturally. It is a clear liquid with a sweet smell that can be detected at low levels. It is also called carbon chloride methane tetrachloride perchloromethane tetrachloroethane or benziformCarbon tetrachloride is most often found in the air as a colorless gas.
It is not flammable and does not dissolve in. A multicomponent 3D monolithic electrode was designed that consists of Co single atoms anchorednitrogen sulfur co-doped carbon nanotubes CoSAs-NS-CNTs on carbon cloth CC with ultra-thin MoS 2 nanosheets externally decorated and ultra-small CoS 2 nanodots internally confined MoS 2 email protected 2 CC to form a hybrid heterojunction electrode with double interfaces as vectorial. Sodium formate chemical shifts.
190 and 844 ppm respectively. To each tube 50 µL of the stock solution and 3 µL of TMS1 were added. The solvent chemical shifts3 were obtained from the spectra containing the solutes and the ranges of chemical shifts 1 For recommendations on the publication of NMR data see.
Ignites steel at 100 C in the presence of soot rust carbon or other catalysts. Ignites dry steel wool at 50 C. Reacts as either a liquid or gas with alcohols explosion molten aluminum explosion silane explosion bromine pentafluoride carbon disulfide explosion catalyzed by iron 1-chloro-2-propyne excess chlorine causes an explosion dibutyl phthalate explosion at 118 C.
Expanding organofluorine chemical space. The design of chiral fluorinated isosteres enabled by IIIIII. This study demonstrates the viability of the thiolatedisulfide redox couple in AZIB applications and provides an in-depth study on the electrochemical mechanism of Zn-thiolates electrode materials.
From the themed collection. 2021 Chemical Science HOT Article Collection. The mechanisms of adhesion by the participation of chemical bonding are the oldest and the most frequently proposed because chemical bonding forms reliable easy to explain connections between substrate and adherent.
The covalent bonds have high energy eg CC 347 CO 358 CN 305 CS 259 NO 201 and OO 146 kJmol and they require substantial energy. CCl4 - carbon tetrachloride CS2 - carbon disulfide CDCl3 - Deuteriochloroform C6D6 - Hexa deuteriobenzene D2O - Deuterium oxide 14. Powerpoint Templates Page 14 Chemical shift A chemical shift is defined as the difference in parts per million ppm between the resonance frequency of the observed proton and tetramethylsilane TMS hydrogens.
TMS is the most common reference. We would like to show you a description here but the site wont allow us. Carbon-coated Na 2 Fe 05 Mn 05PO 4 F 6 wt of carbon also showed a reversible capacity of 110 mA h g 1 confirming the feasibility of Na ion migration intoout of the tunnel structure.
180 Polarization of the Mn redox is clearly greater than that of the Fe 23 redox which is similar to those observed in LiFePO 4 and LiMnPO 4. The Journal of the American Chemical Society JACS founded in 1879 is the flagship journal of the American Chemical Society and the worlds preeminent journal in all of chemistry and interfacing areas of science. This periodical is devoted to the publication of fundamental research papers and publishes approximately 19000 pages of Articles Communications and Perspectives a year.
Sulfur molten appears as a pale yellow crystalline solid with a faint odor of rotten eggsInsoluble in waterA fire and explosion risk above 450 F. Transported as a yellow to red liquid. Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier.
NPTEL Chemical Mass Transfer Operation 1. Carbon-disulfide - acetone 610 mole CS 2 3925 oC 1 atm and Benzene - water 296 mole H 2 O 6925 oC 1 atm are minimum-boiling azeotropes. Hydrochloric acid - water 111 mole HCl 110 oC 1 atm.
Acetone - chloroform 655 mole chloroform 645 oC 1 atm are the examples of Maximum-boiling azeotropes. The Figure 55 shows two. In the chemical industry a number of chemical reactions are mediated by adsorbed inorganic compounds on carbon.
An example of such a catalyst is palladium on activated carbon. When activated carbon is soaked in a dilute solution of palladium chloride and hydrochloric acid for several hours and then washed the palladium ions adsorb on the activated carbon and the chloride ions are completely. An example of a chemical change is Afreezing water B.
Breaking a glass Csublimation of carbon dioxide D. Digestion of food 48. Acetic acid is classified as weak acid because it Adoes not ionize in water B.
Does not neutralize bases C. Slightly ionize in water D. React rapidly with zinc to produce hydrogen 49.
The carbon-carbon-carbon bond angle in H2CCCH2 is A. 109 C120 D180 19. An atomic face-centered cubic crystal is 392 angstrom on an edge and has a.
Even low-level sources of ignition such as hot plates static discharge from clothing steam lines or other hot surfaces provide a sufficiently energetic ignition source for the most flammable substances in general laboratory use such as diethyl ether and carbon disulfide see Chapter 4 section 4D13 and Vignette 63. Flammable substances that require low-temperature storage should be. These are the basic chemical names or mineral names along with chemical composition and sometimes when available manufacturing details.
I have also included CAS numbers when I can fine them. Sometimes multiple names are given because chemical names can be stated in different ways the different chemical names can be an indication of the different manufacture methods. Carbon disulfide Z373-1968 20 ppm.
1 ppm ST 12 ppm C 30 ppm. 1 ppm ST 10 ppm. Carbon tetrachloride Z3717-1967 10 ppm.
5 min in any 4 hr. 2 ppm ST 10 ppm C 200 ppm. Ca ST 2 ppm 60-min See Appendix A.
5 ppm ST 10 ppm. Chromic acid and chromates Z37-7-1971 1 mg10m 3 See Chromium VI compounds in Annotated Table Z-1.