
Computed by XLogP3 30 PubChem release 20210507 Hydrogen Bond Donor Count. This is determined with the concept of electro-negativity.
A chemical property describes the ability of a substance to undergo a specific chemical change.
Chemical property of nitrogen. To identify a chemical property we look for a chemical change. A chemical change always produces one or more types of matter that differ from the matter sspresent before the change. The formation of rust is a chemical change because rust is a different kind of matter than the iron oxygen and water present before the rust formed.
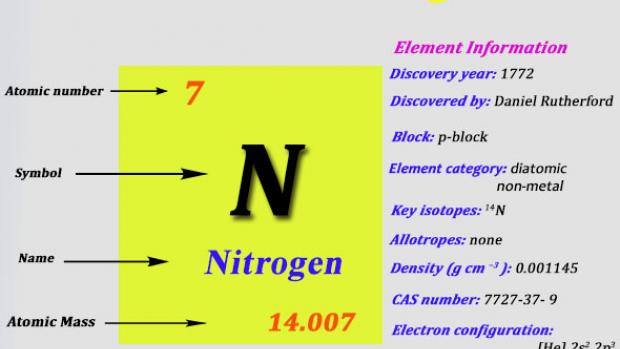
The explosion of nitroglycerin is a chemical change because. Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772.
Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean. A chemical property describes the ability of a substance to undergo a specific chemical change.
To identify a chemical property we look for a chemical change. A chemical change always produces one or more types of matter that differ from the matter present before the change. The formation of rust is a chemical change because rust is a different kind of matter than the iron oxygen.
Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms.
The two extreme cases of chemical bonds are. Bond in which one or more pairs of electrons are shared by two atoms. Nitrogen is important to the chemical industry.
It is used to make fertilisers nitric acid nylon dyes and explosives. To make these products nitrogen must first be reacted with hydrogen to produce ammonia. This is done by the Haber process.
150 million tonnes of ammonia are produced in this way every year. Nitrogen gas is also used to provide an unreactive atmosphere. It is used in this.
This is the property of these elements which leads. One gas one solid two metalloids one metal and one element with unknown chemical properties. All the elements in the group are solids at room temperature except for nitrogen which is gaseous at room temperature.
Nitrogen and bismuth despite both being pnictogens are very different in their physical properties. For instance at STP. This refers to the choking property of nitrogen gas as opposed to air which contains oxygen as well as nitrogen.
One way of remembering the identity of the pnictogen group is to remember the word starts with the symbols of two of its elements P for phosphorus and N for nitrogen. The element family may also be termed pentels which refers both to the elements formerly belonging to. Property Name Property Value Reference.
Computed by PubChem 21 PubChem release 20210507 XLogP3-AA. Computed by XLogP3 30 PubChem release 20210507 Hydrogen Bond Donor Count. Computed by Cactvs 34818 PubChem release 20210507 Hydrogen Bond Acceptor Count.
Computed by Cactvs 34818 PubChem. To identify a chemical property we look for a chemical change. A Copper and nitric acid undergo a chemical change to form copper nitrate and brown gaseous nitrogen dioxide.
B During the combustion of a match cellulose in the match and oxygen from the air undergo a chemical change to form carbon dioxide and water vapor. C Cooking red meat causes a number of chemical. Nitrogen trifluoride is preferred to F2 in high-power chemical lasers because of its ease of handling at ambient temperatures.
Minor uses have been mentioned in the literature. Plasma etching of semiconductor materials production of perfluoroammonium salts a proposed gas filler to increase the life and brightness of lamps and as an. Nitrogen in urea makes it water soluble a highly desired property in this application.
Urea was found to be useful for commercial and industrial applications in the production of some types of plastics animal feed glues toilet bowl cleaners dish washing machine. Chemical Engineering Progress November 2011. Nitrogen is used for a variety of applications including purging pressure transferring inerting and blanketing.
Nitrogen blanketing is the process of applying the gas to the vapor space of a container to control its composition. The benefits of nitrogen blanketing include improved process safety better product quality and longer. Chemical bond polarity is the concept that explains the property of sharing an electron between two elements.
Covalent bond between the elements can be either polar or non-polar. This is determined with the concept of electro-negativity. If the electrons are shared equally between the atoms then its a non-polar covalent bond.
If one of the atom is electronegative it has more tendency to. Liquid nitrogen cryotherapy is a simple and effective treatment option. The risk of intense swelling due to the lax skin tissue in the eyelid is the reason this treatment is generally avoided in XP.
However Labandeira et al. Published a case series of. High Quallity 1 4-B Utanediol BDO CAS 110-63-4.
Best Price High Quality 99 Bdo Liquid Factory Supply 1 4-Butanedi CAS 110-63-4. 99 Bdo Liquid Factory Supply 1 4-Butanedi CAS 110-63-4. Significant sources of VOC are chemical plants gasoline pumps oil-based paints autobody shops and print shops.
Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants industrial furnaces and boilers and motor vehicles. Are high ambient ozone concentrations found only in heavily urbanized areas.
Many people mistakenly believe that. Nitrogen frozen to lock in freshness. Chemical and antibiotic free.
Maine Lobster Tails 10oz - 12oz. As low as 4899. Working safely with hazardous chemicals requires proper use of laboratory equipment.
Maintenance and regular inspection of laboratory equipment are essential parts of this activity. Many of the accidents that occur in the laboratory can be attributed to improper use or maintenance of laboratory equipment. This chapter discusses prudent practices for handling equipment used frequently in.
Except for Nitrogen all the other elements have allotropes. The valence shells of the p-Block elements have a configuration of ns 2 np 3. So the elements here can either lose 5 electrons or gain 3.
The common oxidation states of these elements are -3 3 and 5. Nitrogen is a chemical element with atomic number. Electronegativity symbol χ is a chemical property that describes the tendency of an atom to attract electrons towards this atom.
For this purposes a dimensionless quantity the Pauling scale symbol χ is the most commonly used. The electronegativity of Potassium is. In general an atoms electronegativity is affected by.
Nitrogen-based flame retardants are used in nylons polyolefins polyurethane foams and fire-resistant paints textiles and wallpapers. Inorganic flame retardants and mineral compounds. Various inorganic and mineral compounds are combined with bromine phosphorus or nitrogen and used as flame retardants or as elements of flame retardant.
A two-dimensional nitrogen-rich carbon nitrogen C 3 N 5 material was prepared via a facile high temperature thermal polymerizationFor the first time the C 3 N 5 was used as fiber coating of solid-phase microextraction SPME to extract and preconcentrate polychlorinated biphenyls PCBs before gas chromatography GC analysis. The X-ray diffraction N 2 adsorption-desorption Fourier. A chemical element such as boron carbon or nitrogen that lacks metal properties and is capable of forming anions acid oxides acids and stable hydrogen compounds.
What are non-metal and metal. Substance whether solids liquids or gases can be metals. The sensitivity of a particular soil property to heating is also important.
In general changes in soil chemical properties are directly related to the changes in OM described earlier. However some soil physical properties are also dependent on soil OM while others are not for example clay content. Soil microorganisms are probably most.
The Chemical Identifier fields include common identification numbers the. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia hydrogen hydrogen sulfide or methane. Mixtures of hydrogen carbon monoxide or methane and oxygen difluoride are exploded when a spark is discharged Mellor 2 Supp.