
It is freely soluble in water and practically insoluble in ethanol. It is freely soluble in water and practically insoluble in ethanol.
The solid dissolves readily in water and its solutions have a salt-like taste.
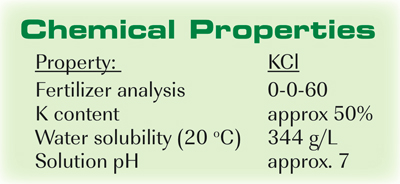
Chemical properties of potassium chloride. Potassium Chloride is a metal halide composed of potassium and chloride. Potassium maintains intracellular tonicity is required for nerve conduction cardiac skeletal and smooth muscle contraction production of energy the synthesis of nucleic acids maintenance of blood pressure and normal renal function. This agent has potential antihypertensive effects and when taken as a nutritional.
Since potassium chloride is completely ionized into K and Cl ions in water the resulting aqueous solution exhibit high values of electrical conductivity. The reduction of potassium chloride into metallic can be achieved with the help of metallic sodium despite the lower electropositivity of sodium when compared to potassium. This is achieved by heating the KCl with.
Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water and its solutions have a salt-like taste.
Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer in medicine in scientific applications. Potassium chloride supplements are used to prevent and treat low blood levels of potassium this is also called hypokalemia.
Available as extended-release capsules and tablets sprinkles an oral solution and in a sterile solution for intravenous administration. Extended-release supplements release potassium chloride slowly over a period of time. The dosage of potassium.
Potassium - K Chemical properties of potassium - Health effects of potassium - Environmental effects of potassium. Electronegativity according to Pauling. 086 gcm-3 at 0 C.
Electronic shell Ar 4s 1. Potassium chloride is used to prevent or to treat low blood levels of potassium hypokalemia. Potassium levels can be low as a result of a disease or from taking certain medicines or after a prolonged illness with diarrhea or vomiting.
The potassium content of the Dead Sea is estimated at approximately 17 percent potassium chloride and many other salty bodies of water are rich in potassium. The waste liquors from certain saltworks may contain up to 40 grams per litre of potassium chloride and are used as a source of potassium. Potassium is an alkali metal and is a part of group 1.
Its symbol is K taken from its Latin name Kalium. Its atomic number is 19 and atomic weight is 39098u. It is white with a silvery shine or luster.
It is soft at room temperature. Potassium is a fine conductor of electricity and heat. Monopotassium phosphate MKP also potassium dihydrogenphosphate KDP or monobasic potassium phosphate KH2PO4 is a soluble salt of potassium and the dihydrogen phosphate ion.
It is a source of phosphorus and potassium as well as a buffering agent. It can be used in fertilizer mixtures to reduce escape of ammonia by keeping pH low. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash the ashes of plants from which its name derives.
Chlorine to produce metal chloride. Bromine to produce metal bromide. To predict the properties of rubidium caesium and francium.
Rubidium caesium and francium are placed below potassium in Group 1 of the Periodic Table. Hence rubidium caesium and francium are expected to react with water oxygen chlorine or bromine in a similar way as potassium but these reactions are more. Because potassium chloride is more soluble in water than sodium chloride certain rock salt depositssuch as those at Stassfurt Germanywere covered by a layer of potassium chloride.
In order to gain access to the sodium chloride the potassium salt important as a fertilizer is removed first. Potassium chloride extended-release capsules USP 10 mEq are electrolyte replenishers. The chemical name of the active ingredient is potassium chloride USP and the structural formula is KCl.
Potassium chloride USP occurs as a granular crystalline powder. It is freely soluble in water and practically insoluble in ethanol. The inactive ingredients are ethylcellulose FDC red 3 FDC yellow.
The chemical name of the active ingredient is potassium chloride and the structural formula is KCl. Potassium chloride USP occurs as a white crystalline powder. It is odorless and has a saline taste.
It is freely soluble in water and practically insoluble in ethanol. Potassium chloride is a tablet formulation not enteric coated or wax matrix containing individually microencapsulated. The chemical formula of a compound is a symbolic representation of its chemical composition.
Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. For example the chemical formula of water which is H. Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure.
The chemical symbol for Potassium is K. Potassium was first isolated from potash the ashes of plants from which its name derives. In the periodic table potassium is one of the alkali metals.
All of the alkali. Potassium Chloride Extended Release Tablets may be used alone or with other medications. Potassium Chloride Extended Release Tablets belongs to a class of drugs called Electrolyte Supplements Parenteral.
It is not known if Potassium Chloride Extended Release Tablets is safe and effective in children younger than 1 month of age. Calcium chlorides exothermic properties are exploited in many self-heating food products where it is activated mixed with water to start the heating process providing a nonexplosive dry fuel that is easily activated. In brewing beer calcium chloride is sometimes used to correct mineral deficiencies in the brewing water.
It affects flavor and chemical reactions during the brewing. The variety of combinations of epoxy resins and reinforcements provides a wide latitude in properties obtainable in molded parts. The table below can be used as an indication of epoxy resistance to chemical compounds.
Always check the chemical resistance with the epoxy manufacturer. Chemical Product Epoxy Resistance to Chemical Product. Acetic Acid 20 Excellent.
The chemical name is potassium chloride and the structural formula is KCl. Potassium chloride USP is a white granular powder or colorless crystals. It is odorless and has a saline taste.
Its solutions are neutral to litmus. It is freely soluble in water and insoluble in alcohol. Hydrogenated vegetable oil magnesium stearate polyethylene glycol polyvinyl alcohol.
The University of Georgia Agricultural and Environmental Services Laboratories offer soil salinity testing to help farmers and the general public diagnose and manage problems associated with soil salinity. By definition a saline soil contains excess soluble salts that reduce the growth of most crops or ornamental plants. This publication discusses soil salinity testing data interpretation.