
The vapors are slightly heavier than air and may explode if ignited. The vapors are slightly heavier than air and may explode if ignited.
This distinction is required on the labels of research chemicals and is what exempts them from regulation under parts 100-740 in.
Chemical properties of methanol. However the detection of methanol in the blood and exhaled air of healthy volunteers suggests that methanol may be a chemical with specific functions rather than a metabolic waste product. Using a genome-wide analysis of the mouse brain we demonstrated that an increase in blood methanol concentration led to a change in the accumulation of mRNAs from genes primarily involved in. Methanol has chemical properties which allow it to lower the freezing point of a water-based liquid and increase its boiling point.
These attributes lead methanol to be used as an antifreeze in windshield washer fluid to keep the cleaning fluid from freezing. It is also injected in natural gas pipelines where it lowers the freezing point of water during oil and gas transport. Core of this report is the explanation of the main physical and chemical properties of methanol as well as how these properties affect the different types of existing engines in the market.
SGS has performed an analysis of physical properties on nineteen samples of gasoline methanol ethanol methyl tert-butyl ether MTBE and tertbutyl alcohol TBA in different blending ratio. Methanol CH 3-OH is a colorless fairly volatile liquid with a faintly sweet pungent odor similar but somewhat milder and sweeter than ethanol. Methanol is toxic and may cause blindness.
The vapors are slightly heavier than air and may explode if ignited. Methanol is used to make chemicals to remove water from automotive and aviation fuels as a solvent for paints and plastics and as. The modern method of preparing methanol is based on the direct combination of carbon monoxide gas and hydrogen in the presence of a catalyst.
Increasingly syngas a mixture of hydrogen and carbon monoxide derived from biomass is used for methanol production. Pure methanol is an important material in chemical synthesis. The Physical Property fields include properties such as vapor pressure and boiling point as well.
Chemical PAC-1 PAC-2 PAC-3. Methyl alcohol 67-56-1 530 ppm. LEL 55000 ppm.
Indicates value is 10-49 of LEL. DOE 2016 Regulatory Information. What is this information.
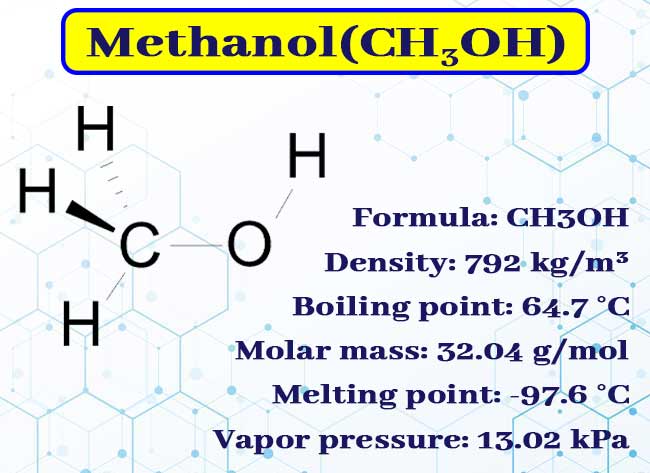
The Regulatory Information fields include information from the US. Methanol also known as methyl alcohol amongst other names is a chemical and the simplest alcohol with the formula C H 3 O H a methyl group linked to a hydroxyl group often abbreviated MeOH. It is a light volatile colourless flammable liquid with a distinctive alcoholic odour similar to that of ethanol potable alcohol.
A polar solvent methanol acquired the name wood alcohol because. The chemical formula of Methanol is CH 3 OH. Since its chemical structure is a linkage between a methyl CH 3 group and a hydroxyl group OH it is sometimes also written as MeOH where Me stands for Methyl.
The molecular weight or molar mass of Methanol is 3204 gmol. The various forms in which the molecular structure of Methanol can be represented are given below. Research chemicals are chemical substances used by scientists for medical and scientific research purposes.
One characteristic of a research chemical is that it is for laboratory research use only. A research chemical is not intended for human or veterinary use. This distinction is required on the labels of research chemicals and is what exempts them from regulation under parts 100-740 in.
Methanol methyl alcohol carbinol wood alcohol wood naptha or wood spirits is a chemical compound with chemical formula CH 3 OH. Thermophysical properties for temperatures ranging -50-150 o C are indicated in the table below. For full table with Liquid Viscosity Vapor Viscosity Vapor Pressure Vapor Specific Heat and Liquid Surface Tension - rotate the screen.
Chemical Properties Of Acetic Acid. The bonding behavior of acetic acid is due to its constituent functional groups. Much of the chemical bonding character of acetic acid is due to its carboxyl group and accompanying OH group.
The hydroxyl group allows molecules of acetic acid to engage in hydrogen bonding. The polar hydrogen end of the hydroxyl group will attract the polar. The properties of the diatomic form suggest that six electrons bond the atoms and two electrons remain unpaired.
Medical applications of oxygen include use in oxygen tents inhalators and pediatric incubators. Oxygen-enriched gaseous anesthetics ensure life support during general anesthesia. Oxygen is significant in a number of industries that use kilns.
Methanol-13C CH4O CID 11205710 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS. Water is the chemical substance with chemical formula H 2 O one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Let us learn about the physical and chemical properties of water. A glance of earth taken from space will depict it blue. This blue colour is actually water the major part of.
Methanol the simplest alcohol CH3OH is a chemical building block for hundreds of everyday products including plastics paints car parts and construction materials. Methanol also is a clean energy resource used to fuel cars trucks buses ships fuel. Chemical properties can be identified by the changes they produce.
Tarnishing is a reaction of silverwares such as a spoon to oxygen and other gases. Hence tarnishing is a chemical property of. Research on Chemical Intermediates publishes current research articles and concise dynamic reviews on the properties structures and reactivities of intermediate species in all the various domains of chemistry.
The journal also contains articles in related disciplines such as spectroscopy molecular biology and biochemistry atmospheric and environmental sciences catalysis. Generally this compound is produced via the reaction between methanol and carbon monoxide carbonylation of methanol. Chemical Properties of Acetic Acid.
The chemical reactions undergone by acetic acid are similar to those of other carboxylic acids. When heated to temperatures above 440 o C this compound undergoes decomposition to yield either methane and carbon dioxide or water and. Predicted Melting Point-98 C JK Scientific 934255 940402 939085 125146 952707 122729.
Colorless liquid with a characteristic pungent odor. Colourless liquid with a characteristic odour OU Chemical Safety Data No longer updated More details. May react violently with acids acid.
Research Chemical 2-Hydroxy-3-phenylpropiophenone CAS3516-95-8 with Safe Shipping 99 liquid moker. Ethyl vanillin 99 white powder 121-32-4 SAIYI Safe delivery 99 powder saiyi. Methanol CH 3 OH also known as wood alcohol is considered an alternative fuel under the Energy Policy Act of 1992As an engine fuel methanol has chemical and physical fuel properties similar to ethanolMethanol use in vehicles has declined dramatically since the early 1990s and automakers no longer manufacture methanol vehicles in the United States.
Methanol Version 17 Revision Date. 100000002748 3 20 Methanol P370 P378 In case of fire. Use dry sand dry chemical or alcohol-resistant foam for extinction.
P403 P233 Store in a well-ventilated place. Keep container tightly closed. P403 P235 Store in a well-ventilated place.
Chemical Properties of Ethanoic Acid Reactions of Ethanoic Acid Esterification Reaction. When any carboxylic acid reacts with any alcohol it leads to the formation of another class of chemical compounds named esters. This reaction which leads to the formation of esters is known as esterification reaction.
Given below is an example presented. Causes ignition and a mild explosion when bubbled through cold methanol. Explodes or ignites if mixed in excess with ammonia and warmed.
Causes ignition in contact with hydrazine hydroxylamine and calcium nitride. Forms explosive nitrogen trichloride from biuret contaminated with cyanuric acid. Readily forms an explosive N-chloro derivative with aziridine.
Ignites or explodes with arsine. Methanol and acetonitrile have different chemical properties. Methanol is a protic solvent whereas acetonitrile is a non-protic solvent so we know that their elution behavior will differ.
If adequate separation cannot be obtained with an acetonitrile-based mobile phase switching to a methanol-based mobile phase to change the elution order is one useful possibility for method development.