
1810-3 gcm-3 at 20C. Using Two-Dimensional Lithium Fluoride as a Hydrogen Storage Solution.
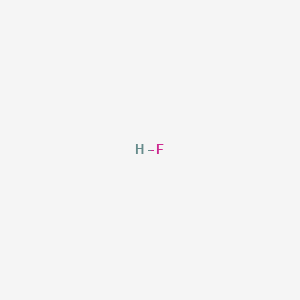
Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
Chemical properties of hydrogen fluoride. Hydrogen fluoride is a chemical compound with the chemical formula H FThis colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acidIt is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers eg. HF is widely used in the petrochemical. Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid Colorless gas or fuming liquid below 67F with a strong irritating odor.
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. For all practical purposes they are considered the same chemical. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
It is an intermediate for many chemical reactions and syntheses. It is used. Hydrogen is the chemical element with the symbol H and atomic number 1.
Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2It is colorless odorless non-toxic and highly combustibleHydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. Chemical properties of fluorine - Health effects of fluorine - Environmental effects of fluorine.
Electronegativity according to Pauling. 1810-3 gcm-3 at 20C. 0136 nm -1.
Electronic shell He 2s 2 2p 5. Having a chemical formula of F fluoride ion is the simplest inorganic monatomic anion of fluorine with basic properties. It is considered a trace element.
Fluoride ions are found in various minerals but are only present in trace amounts in waterIts various salt forms and minerals play numerous roles as chemical reagents industrial chemicals and commercial products. In this quiz youll be shown all 118 chemical symbols and youll need to choose the name of the chemical element that each one represents. The isolation of fluorine was for a long time one of the chief unsolved problems in inorganic chemistry and it was not until 1886 that the French chemist Henri Moissan prepared the element by electrolyzing a solution of potassium hydrogen fluoride in.
Using Two-Dimensional Lithium Fluoride as a Hydrogen Storage Solution. The research published in Applied Surface Science has explored the use of two-dimensional lithium fluoride monolayers to provide efficient storage of hydrogen gas in fuel cells. Both the blende phases and hexagonal monolayer phases of lithium fluoride were analyzed for their properties such as spin.
Astatine is placed below iodine in group 7. Use the information to predict the reaction of astatine with hydrogen. Astatine should react very slowly with hydrogen even when heated.
Chlorine - chlorine - Physical and chemical properties. Chlorine is a greenish yellow gas at room temperature and atmospheric pressure. It is two and a half times heavier than air.
It becomes a liquid at 34 C 29 F. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat andafter severe exposureedema filling with fluid. The hydrogen atoms are attached to one side of the oxygen atom resulting in a water molecule having a positive charge on the side where the hydrogen atoms are and a negative charge on the other side where the oxygen atom is.
Since opposite electrical charges attract water molecules tend to attract each other making water kind of sticky As the right-side diagram shows the side with. For all practical purposes they are considered the same chemical. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
It is an intermediate for many chemical reactions and syntheses. It is used to remove and inhibit rust and to etch polish and frost glass. It is used in the manufacture of silicon semiconductor.
What are the chemical properties of water. Chemical properties of water. The chemical formula of a molecule of water is H2O.
Two atoms hydrogen H2 linked to one atom oxygen O. The atom electrons particles with a negative charge establish links between themselves. Oxygen is more able to keep them close to it than hydrogen.
Dioxygen difluoride is a terrifying chemical which also goes by the charming nickname FOOF because it is two fluorine atoms joined by two oxygen atoms. In 1962 chemist AG. Streng published a paper called The Chemical Properties of Dioxygen Difluoride Although the title may not be thrilling Strengs experiments certainly were.
Ical resistance properties of its products or the toxicological effects of chemicals reacting with them. Nothing appearing here should be considered as a recommendation for any use that would infringe patent rights or an endorsement for the use of any particular material or chemical or any class of materials or chemicals. Chemical Substance Concentration TemperatureºF Chemical Substance.
Effects of Hydrogen Bonding on Elements Association. The molecules of carboxylic acids exist as dimer because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formula.
In aqueous solution HF dissociates and gives the difluoride ion instead of fluoride ion. This is due. Chemical compounds are formed when elements are joined by chemical bonds.
These bonds are so strong that the compound behaves like a single substance. Compounds have their own properties that are unique from the elements they are made of. A compound is a type of molecule with more than one element.
You can go here to learn more about molecules and compounds. Also known as. Hydrogen fluoride hydrofluoride hydrogen monofluoride fluorhydric acid.
Although it is highly corrosive hydrofluoric acid is considered a weak acid because it doesnt usually dissociate completely. The acid will eat glass and metals so HF is stored in plastic containers. If spilled on skin hydrofluoric acid passes through soft tissue to attack bone.
HF is used to make. The chemical element Calcium Ca atomic number 20 is the fifth element and the third most abundant metal in the earths crust. The metal is trimorphic harder than sodium but softer than aluminiumA well as beryllium and aluminium and unlike the alkaline metals it doesnt cause skin-burns.
It is less chemically reactive than alkaline metals and than the other alkaline-earth.