Ions of Some Nonmetals Groups IVA - VIIA. The charge on the anion is the group number minus eight.
First FOR ALL EQUILIBRIUM PROBLEMS YOU MUST.
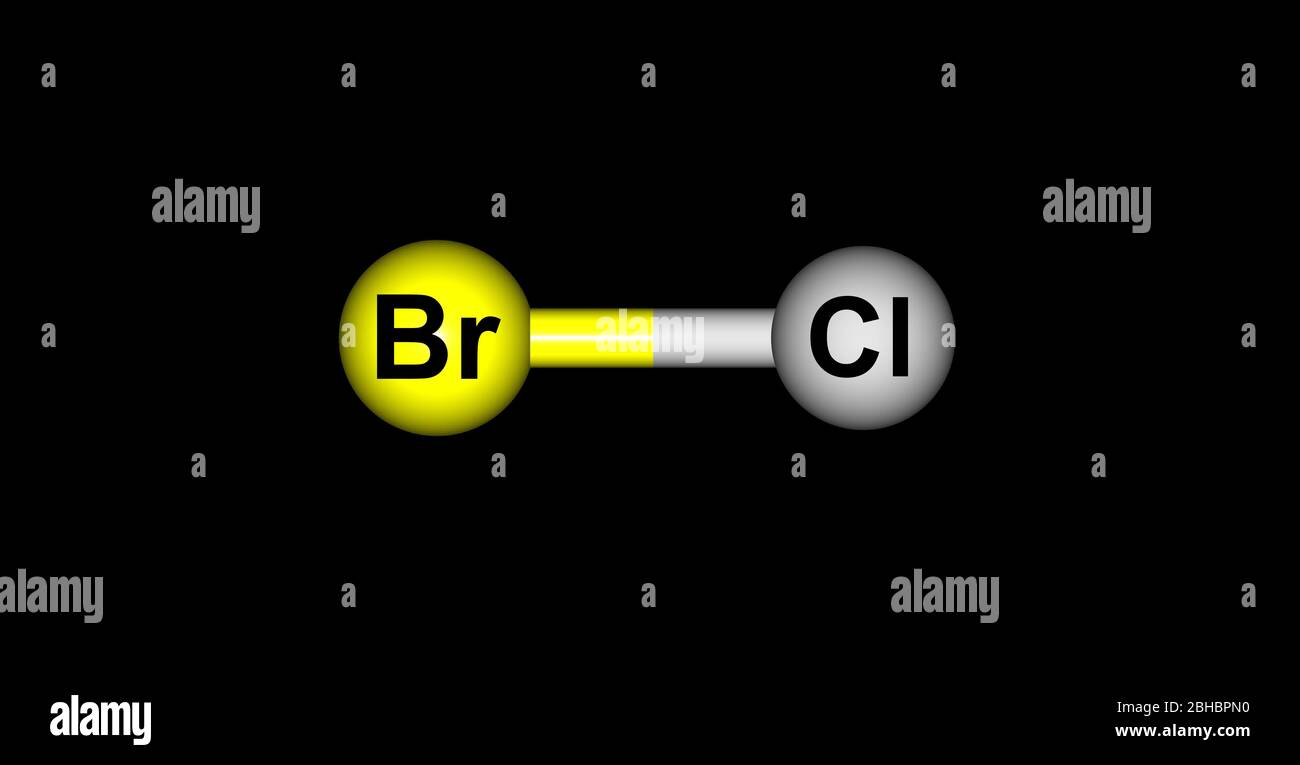
Chemical name of brcl. These new orbitals formed are called hybrid orbitals and serve the purpose of chemical bonding in valence bond theory. Observing the hybridization of the bromine atom in BrCl3 we find that bromineBr atom is sp3d hybridized. In sp3d hybridization one s orbital combines with three p orbitals and one d orbital having almost the same energy to form 5 identical hybrid.
Long before chemists knew the formulas for chemical compounds they developed a system of nomenclature that gave each compound a unique name. Today we often use chemical formulas such as NaCl C 12 H 22 O 11 and CoNH 3 6 ClO 4 3 to describe chemical compoundsBut we still need unique names that unambiguously identify each compound. CHEMICAL EQUILIBRIUM Name Last Ans.
First FOR ALL EQUILIBRIUM PROBLEMS YOU MUST. 1 Write all equilibrium equations 2 Write all equilibrium concentrations 3 Write all equilibrium expressions SET A. A What is the equilibrium Constant expression for the reaction.
3 FeS 4 H20 g s 4 H2 g b The equilibrium constant Kc for the reaction. 2 NOCI 2 NO g C12 g What. 3 BrCl 4 NH3 NBr3 3 NH4Cl.
Chemical Elements Periodic Table Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter. Other names CarbonIV bromide. 558-13-4 3D model.
Abbreviations R-10B4 citation needed Beilstein Reference. This is a list of common chemical compounds with chemical formulae and CAS numbers indexed by formula. Bromine monofluoride bromine fluoride.
7726-95-6 Br 2 O 5. It is a substance that will slow down the speed of chemical reaction A. Substrate CHEMICAL ENGINEERING REVIEW ANALYTICAL CHEMISTRY 2.
A solution containing 253 mL of 01065 N HCl is added to one containing 922 mL of 02715 M H 2SO 4 and 50 mL of 100 N KOH are added. What is the IUPAC name of the molecule shown above. 1-aminopentan-3-ol Question 5 The metabolic process that produces water is A.
The digestion of fats. The hydrolysis of starch. The breakdown of protein into amino acids.
Question 6 Which one of the following pairs of. Main-Group Nonmetals Groups IVA VA VIA and VIIA. Group IVA VA VIA and VIIA nonmetals tend to form anions by gaining enough electrons to fill their valence shell with eight electrons.
The charge on the anion is the group number minus eight. The anion is named by taking the element stem name and adding the ending -ide. Ions of Some Nonmetals Groups IVA - VIIA.
Assign formal charges to each atom in the interhalogen molecule BrCl 3. Assign one of the electrons in each BrCl bond to the Br atom and one to the Cl atom in that bond. Assign the lone pairs to their atom.
Now each Cl atom has 6 unshared electrons and 2 bonding electrons and the Br atom has 4 unshared electrons and 6 bonding electrons. Subtract this number from the number of. Structural isomers as their name implies differ in their structure or bonding which are separate from stereoisomers that differ in the spatial arrangement of the ligands are attached but still have the bonding properties.
The different chemical formulas in structural isomers are caused either by a difference in what ligands are bonded to the central atoms or how the individual ligands are. The standard state of a chemical substance is its phase solid liquid gas at 250 C and one atmosphere pressure. This temperaturepressure combo is often called room conditions Determine the specific heat capacity of an alloy that requires 593 kJ to raise the temperature of 1500 g alloy from 298 K to 398 K.
Name the 7 diatomic elements. H₂ N₂ O₂ F₂ Cl₂. Physical and Chemical Treatments.
Various treatments applied to raw water to remedy undesirable characteristics eg color taste odor or turbidity may affect the ultimate microbiological quality of the finished water. Microorganisms may be physically removed or the disinfectant demand of the water altered. Presedimentation to remove suspended matter coagulation with alum or other agents.