Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure. Sodium chloride NaCl and magnesium oxide MgO.
Mercury is commonly known as quicksilver and was formerly named hydrargyrum.
Chemical formula for mercury. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. An ionic compound is composed of a metal and a non-metal. Sodium chloride NaCl and magnesium oxide MgO.
The transfer of electrons between metals and non-metals produces charged particles called ions. Metals lose electrons to produce positve ions called cations. Na Mg 2 Non.
A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound. Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O. Similarly sulfuric acid has a chemical formula H₂SO₄ which means that in this compound the.
Each chemical substance has a specific chemical composition so these chemical substances have their own chemical formula. Lets take a look at. Chemical formula plays an important role in understanding different concepts of chemistry.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Chemical formulas can be.
Up to 1 mgm 3. APF 10 Any chemical cartridge respirator with cartridges providing protection against the compound of concern APF 10 Any supplied-air respirator. Up to 25 mgm 3.
APF 25 Any supplied-air respirator operated in a continuous-flow mode. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides.
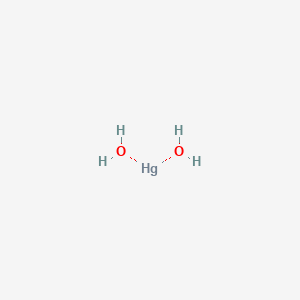
It is characterized as a heavy silvery-white metallic liquid at room temperature that is odorless. Although its formula H 2 O seems simple water exhibits very complex chemical and physical properties. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds.
MercuryI chloride is the chemical compound with the formula Hg 2 Cl 2. Also known as the mineral calomel a rare mineral or mercurous chloride this dense white or yellowish-white odorless solid is the principal example of a mercuryI compound. It is a component of reference electrodes in electrochemistry.
The name calomel is thought to come from the Greek καλός beautiful. Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure. The chemical symbol for Mercury is Hg.
Mercury is commonly known as quicksilver and was formerly named hydrargyrum. Mercury is a heavy silvery d-block element mercury is the only metallic element that is liquid at standard conditions for temperature and pressure. A chemical formula is a way of expressing information about the proportions of atoms that constitute a particular chemical compound using the standard abbreviations for the chemical elements and subscripts to indicate the number of atoms involved.
For example water is composed of two hydrogen atoms bonded to one oxygen atom. The chemical formula is H 2 O. In the case of non-stoichiometric.
Sodium ions get deposited at the mercury cathode forming a sodium amalgam. Chlorine ions move towards the anode and exit the cell from the top. Reaction at the anode.
2 C l C l 2 2 e. Reaction at the cathode. 2 N a 2 e 2 N a.
The amalgam formed is then transferred to another chamber called denuder. In the denuder it is treated with water to obtain. It is used in nuclear reactors as a neutron moderator.
Na 2 S 2 O 35H 2 O. It is used for both film and photographic paper processing. Iron lll Oxide Ferric Oxide.
Fe 2 O 3. It is used to produce pigments preparations for heavy media. In food products containing milk and milk-derived ingredients except infant formula and sole source nutrition products including meal replacement products.
In the edible portion of all retail fish with six exceptions see the 1 ppm maximum level below. See also advice on canned whitealbacore tuna via the Mercury. Chemical Nomenclature V - Common Name Examples using Mercury.
Chemical Nomenclature VI - Binary Compounds of Two Nonmetals. The Greek Prefix System. Chemical Nomenclature VII - Polyatomic Ions.
Chemical Nomenclature VIII - Polyatomic Ions. WebElements - the most extensive web-based periodic table. For example the element mercury is shown as Hg.
You must not show it as HG hg or hG. Represents an element or compound. Of or relating to chemistry.
Of or relating to the properties or actions of chemicals. Of or relating to chemical weapons. A substance with a distinct molecular composition that is produced by or used in a chemical process.
A drug especially an illicit or addictive one. Eventually after studying chemistry for some time you should be able to look at the formula of a compound and state some chemical property. For example hydrogen has the potential to ignite and explode given the right conditionsthis is a chemical property.
Metals in general have the chemical property of reacting with an acid. Zinc reacts with hydrochloric acid to produce hydrogen gas. The final chemical formula for dinitrogen hexafluoride is N 2 F 6.
Practice with some examples. H 3 and mercuryI Hg 2 2. They all have a 1 charge though technically 2 mercury atoms are bonded together which creates a 2 charge with each mercury cation containing a 1 charge.
The rest of the polyatomic ions have negative charges ranging from -1 to -4. The formula weight often used interchangeably with molecular weight of a compound will be listed in gramsmole gmol on the side of the chemical bottle. If you cannot find the formula weight on the bottle you can search for the compound online to find it.
The formula weight of sodium chloride NaCl 5844 gmol. Hg2Cl2 mercury I chloride 57. KCIO potassium hypochlorite 48.
ZnCH3COO2 zinc acetate 58. Mg3N2 magnesium nitride 49. K2SO4 potassium sulfate 59.
NaMnO4 sodium permanganate 50. Co2SO43 cobalt III sulfate 60. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.
It is naturally available as cinnabar which is a sulfide compound of mercury that produces red vermilion through grinding. Its electric conductance is similar to other metals but heat conductance is very low. Due to the presence of unknown impurities it is certified as lab-grade chemical.
Lab grade chemicals possess reasonable purity but do not comply with any official standard for quality. Heating mercury vapor produces mercuric oxide which is highly irritating to mucous membranes and more likely than elemental mercury vapor to adversely affect the lungs. Elemental mercury reacts with most metals.
Elemental mercury reacts with many acids. Elemental mercury reacts vigorously with ground mixtures of sodium carbide. Mercury reacts with acetylenic.
Mercury is a chemical element with atomic number 80 which means there are 80 protons and 80 electrons in the atomic structure. The chemical symbol for Mercury is Hg. Mercury is commonly known as quicksilver and was formerly named hydrargyrum.
Mercury is a heavy silvery d-block element mercury is the only metallic element that is liquid at. For Water Treatment and Water Distribution. Measurement Conversion Measurement Conversion Measurement Conversion Measurement Conversion 1 ft.
1 MGD 155 cfs 1 grain gal 171 mgL 1 min 60 sec 1 yd. 1 gm 1000 mg 1 hour 60 min 1 m 328 ft. 748 gal 1 kg 1000 gm 1 day 1440 min 1 mi 5280 ft.