The chemical formula for a substance shows how many atoms of each element are present in a molecule or the proportion of atoms of each element. Sulfur dioxide SO2_ 2.
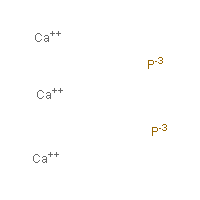
10 V 2S 3 vanadiumIII sulfide.
Chemical formula for calcium phosphide. The chemical formula for a substance shows how many atoms of each element are present in a molecule or the proportion of atoms of each element. The formula can be worked out using the valency. 1305-99-3 Ca 3 PO 4 2.
7758-87-4 Ca 4 PO 4 2 O. 1306-01-0 Ca 5 PO 4 3 F. 12015-73-5 Ca 5 PO 4 3 OH hydroxyapatite.
513-78-0 CdC 2 H 3 O 2 2. 543-90-8 CdC 2 O 4. Chemical formula Synonyms CAS number C 10 H 16 N 2 O 8.
Ethylenediaminetetraacetic acid EDTA 6381-92-6 C 12 H 22 O 11. 57-50-1 C 20 H 25 N 30. Lysergic acid diethylamide LSD 50-37-3 C 123 H 193 N 35 O 37.
Common serum albumin macromolecule 9048-49-1 CaAlH 4 2. 16941-10-9 CaAl 2 O 4. Calciumphosphid Ca3P2 CID 4337964 - structure chemical names physical and chemical properties classification patents literature biological activities.
S Calcium sulfide S Silver sulfide Zn P Zinc phosphide Aluminum oxide Sr I Strontium chloride 4. Circle the symbol for the metal in each of the compounds in Model 2. Which element comes first in the name and formula of the compounds in Model 2the metal or the nonmetal.
Use the table of ions in Model 1 to answer the following questions. In the compound zinc phosphide what is the. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3.
2 calcium chloride Ca and Cl 5 sodium nitride Na and N Formula. 3 aluminum oxide Al and O 6 magnesium phosphide Mg and P Formula. Use Lewis dot structures to show the covalent bonding in the following pairs of elements.
Calcium sulfate 3 C 2 Br 6 dicarbon hexabromide 4 CrCO 3 3 chromium VI carbonate 5 Ag 3 P silver phosphide 6 IO 2 iodine dioxide 7 VO 2 vanadium IV oxide 8 PbS lead II sulfide 9 CH 4 methane 10 N 2 O 3 dinitrogen trioxide Write the formulas of the following chemical compounds. 11 tetraphosphorus triselenide P 4 Se 3 12. Reacts with many metals including aluminum zinc calcium magnesium iron tin and all of the alkali metals to generate flammable hydrogen gas.
Reacts violently with acetic anhydride 2-aminoethanol ammonium hydroxide calcium phosphide chlorosulfonic acid 11-difluoroethylene ethylenediamine ethyleneimine oleum perchloric acid b-propiolactone propylene oxide silver perchlorate. Calcium carbonate 26 NiPO 4 nickel III phosphate 27 Li 2 SO 3 lithium sulfite 28 Zn 3 P 2 zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3. I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate.
Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I Mercury I sulfate N 2 O 3 M Dinitrogen trioxide CdS I. Ca 3 P 2 calcium phosphide. Al 4 C 3 aluminum carbide.
Compounds Containing Polyatomic Ions. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions except there is no need to change to an ide ending since the suffix is already present in the name of the anion. Examples are shown in Table 2.
Names of Some. The bone and tooth enamel in your body contain ionic compounds such as calcium phosphate and hydroxyapatite. Predict the formula of calcium phosphate which contains Ca 2 and PO 4 3-ions.
Calculate the value of x if the formula of hydroxyapatite is Ca x PO 4 3 OH. Click here to check your answer to Practice Problem 5. Write the chemical formula for the following ionic compounds.
Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium hydroxide MgOH 2 iron II chlorate FeC ℓO 3. Formula and the correct name of the chemical compound. The reward for building a correct chemical compound is receiving a number of points based on the number value of the cards played ie.
Sodium chloride would score 2 points 1 point for the cation and one for the anion. Similarly calcium chloride would score 4 points two. Reacts with calcium phosphide incandescently at about 300C.
Reacts violently with phosphorus trioxide Chem. Mixtures with ammonium nitrate or with metal powders can be exploded by shock Kirk and Othmer 8644. Combinations of finely divided sulfur with finely divided bromates chlorates or iodates of barium calcium magnesium potassium sodium or zinc can.
3 P 2O 5 diphosphorus pentoxide. 4 TiSO 4 2 titaniumIV sulfate. 5 FePO 4 ironIII phosphate.
6 K 3N potassium nitride. 7 SO 2 sulfur dioxide. 8 CuOH copperI hydroxide.
9 ZnNO 2 2 zinc nitrite. 10 V 2S 3 vanadiumIII sulfide. Write the formulas for the following chemical compounds.
11 silicon dioxide SiO 2. 12 nickel III sulfide Ni 2S 3. Chemical Formula Nomenclature Practice.
Complete these in lab and on your own time for practice. You should complete this by Sunday. Use the stock form for the transition metals.
Give the formula for the following. Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3.
Naming Binary Ionic Compounds with a Metal That Forms More Than One Type of Cation. If you are given a formula for an ionic compound whose cation can have more than one possible charge you must first determine the charge on the cation before identifying its correct name. For example consider FeCl 2 and FeCl 3.
In the first compound the iron ion has a 2 charge. Calcium sulfide DS Silver sulfide n Zinc phosphide Al Aluminum oxide Strontium chloride Sr 4. Circle the symbol for the metal in each of the compounds in Model 2.
Which element comes first in the name and formula of the compounds in Model 2 or the nonmetal. The name or symbol of the metal comes first. Use the table of ions in Model 1.
P P3 phosphide ion Cl Cl chloride. Formula Name Formula Name NH4. Ca3P2 Calcium phosphide SrI2 Strontium iodide FeCl2 IronII chloride or ferrous chloride 2.
Ionic Compounds Containing a Metal and a Polyatomic Ion. Metals combine with polyatomic ions to give ionic compounds. Name the cation first specifying the charge if necessary then the polyatomic ion as listed in the.
The positive charge more protons versus electrons for a cation is shown by a number and plus sign after the formula. If theres just a plus sign it means the charge is plus 1. Review some examples of cations or positive ions.
Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2 calcium Ca 2 cesium Cs chromium III Cr 3 cobalt Co 2. Chemical formulas for ionic compounds are called ionic formulas The chemical formula for an ionic compound. A proper ionic formula has a cation and an anion in it.
An ionic compound is never formed between two cations only or two anions only. The key to writing proper ionic formulas is simple. The total positive charge must balance the total negative.
In Chapter 9 Chemical Bonds. Thus the formula between calcium ions Ca 2 and nitrate ions NO 3 is properly written CaNO 3 2 not CaNO 32 or CaN 2 O 6. Use parentheses where required.
The name of this ionic compound is simply calcium nitrate. Write the proper formula and give the proper name for each ionic compound formed between the two listed ions. NH 4 and S 2.
A binary covalent compound is composed of two different elements usually nonmetals. For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine.