
11 tetraphosphorus triselenide P 4 Se 3 12. Pain associated with exposure to solutions of HF 1-50 may be delayed for 1-24 hours.
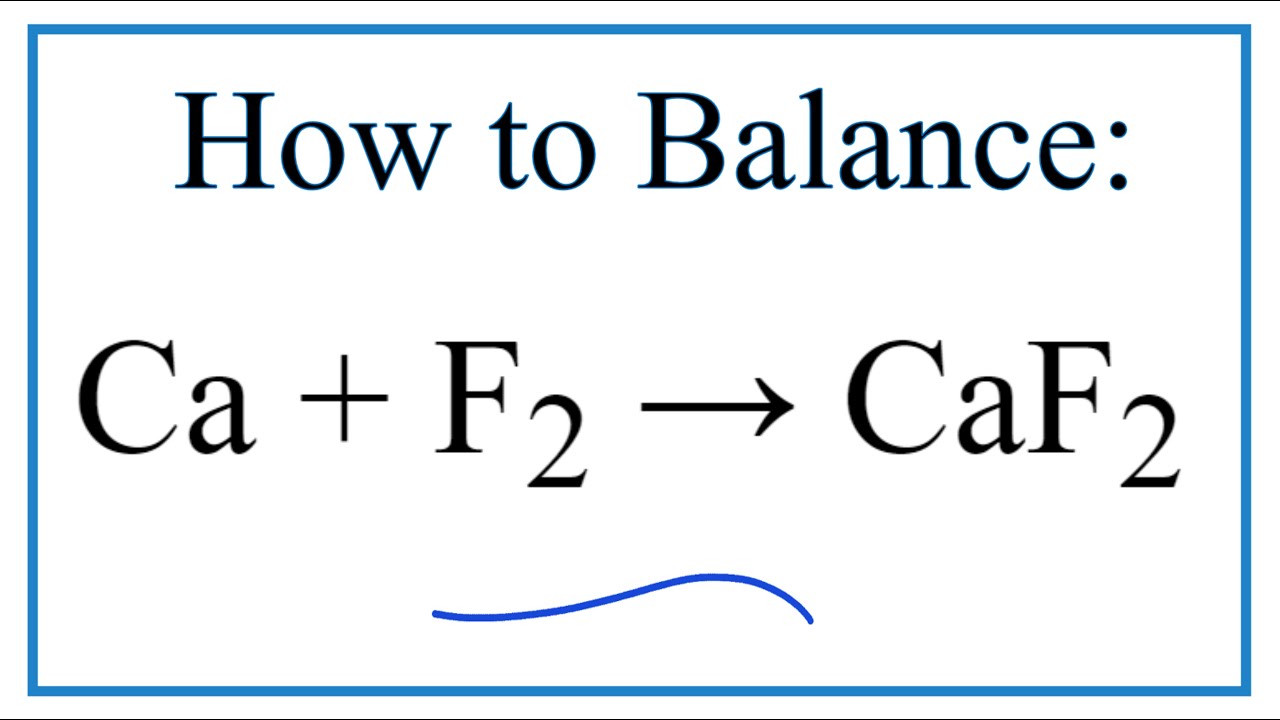
A chemical equation is balanced when the number of atoms of each type on.
Chemical formula for calcium fluoride. Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF 2. It is a white insoluble solid. It occurs as the mineral It is a white insoluble solid.
It occurs as the mineral fluorite also called fluorspar which is often deeply coloured owing to impurities. Gas chromatography was used to measure the maternal and fetal plasma inorganic fluoride values at term in 91 women. They were assigned to one of four groups.
Group A were untreated controls. Group B received a single daily dose of 15 mg of fluoride as calcium fluoride during the final trimester of pregnancy. Group C was given a single dose of 15 mg of fluoride as sodium fluoride and.
The chemical formula of a compound is a symbolic representation of its chemical composition. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. For example the chemical formula of water which is H.
Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. Provided below is a list of the chemical formulas of some common chemical compounds along with their molecular weights. A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound.
Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O. Similarly sulfuric acid has a chemical formula H₂SO₄ which means that in this compound the. Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid Colorless gas or fuming liquid below 67F with a strong irritating odor.
Fluoride ˈ f l ʊər aɪ d ˈ f l ɔːr- is an inorganic monatomic anion of fluorine with the chemical formula F also written F whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals mainly used in the production of hydrogen.
Fluoride is found naturally in soil water and foods. It is also produced synthetically for use in drinking water toothpaste mouthwashes and various chemical products. The chemical formula of an element is a statement of the composition of its molecule in which symbol tells us the element and the subscript tells us how many atoms are present in one molecule.
One molecule of hydrogen element contains two atoms of hydrogen therefore the formula of hydrogen is H 2. For elements that exist as atoms their chemical formulae represent their atoms. Hydrofluoric acid HF differs from other acids because the fluoride ion readily penetrates the skin causing destruction of deep tissue layers including bone.
Pain associated with exposure to solutions of HF 1-50 may be delayed for 1-24 hours. If HF is not rapidly neutralized and the fluoride ion bound tissue destruction may continue for days and result in. For example the molecular formula of sodium fluoride is NaF.
A molecular formula is not a chemical name and it contains no words. Although a molecular formula may imply certain simple chemical structures it is not the same as a full chemical structural formula. Molecular formulas are more limiting than chemical names and structural formulas.
Empirical and Molecular Formulas. Fluoride H 2 O 2 Peroxide C 8 H 10 N 4 O 2 Caffeine NaCl Sodium Chloride C 9 H 8 O 4 Aspirin HCl Hydrochloric Acid ZnNO 3 2 Zinc CO Carbon Monoxide NaOH Sodium Hydroxide NaCN Sodium Cyanide CaCN 2 Calcium Cyanide. BALANCING CHEMICAL EQUATIONS What is a balanced equation.
A chemical equation is balanced when the number of atoms of each type on. Potassium fluoride on calcium fluoride. Potassium fluoride on alumina.
Potassium fluoride ACS reagent. Potassium fluoride on aluminum oxide. Potassium fluorure French Potassium fluoride 40 wt on alumina.
Fluorure de potassium French HSDB 7481. Calcium Nitrate CaOH2 Calcium Hydroxide Ca3PO32 Calcium Phosphate Ca3PO42 Tricalcium Phosphate Ca3N2 Calcium Nitride CaBr2 Calcium Bromide CaC2 Calcium Carbide CaCl2 Calcium Chloride CaCO3 Calcium Carbonate CaF2 Calcium Fluoride CaH2 Calcium Hydride CaI2 Calcium Diiodide CaO Calcium Oxide CaS Calcium Sulfide CaSO4 Calcium Sulfate CBr4. ACP formation is thought to proceed through subnanometer-sized prenucleation clusters with a chemical composition.
The formula of HA shows the sites for atomic substitution. This HA is calcium deficient and carbonated. X calcium substitution with metal cation.
Y phosphate substitution with carbonate. And Z hydroxide substitution with fluoride. Ca 5 PO 4 3 OH 1 Ca 10X.
Describe the chemical reaction based on the chemical equation below. Also explain whether the equation is balanced. Ammonia gas reacts with oxygen gas.
Nitric oxide gas and liquid water are produced. Platinum is used as a catalyst. The equation is unbalanced because the number of hydrogen atoms is not the same on both sides of the equation.
Barium chlorate BaClO32 breaks. Fluoride absorption depends on numerous factors such as stomach pH the chemical formula of consumed fluoride presence of food in the stomach interaction with other food ingredients present in gastrointestinal tract aluminum calcium and magnesium compounds. The unabsorbed fluoride is defecated through feces while the absorbed fluoride is distributed rapidly through the.
Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice. You should complete this by Sunday.
Use the stock form for the transition metals. Give the formula for the following. Sulfur dioxide SO2_ 2.
Sodium thiosulfate Na2S2O3_ 3. Long before chemists knew the formulas for chemical compounds. Predict the formula of calcium phosphate which contains Ca 2 and PO 4 3-ions.
Calculate the value of x if the formula of hydroxyapatite is Ca x PO 4 3 OH. Click here to check your answer to Practice Problem 5. Naming Simple Covalent Compounds Non-metals with non-metals Oxidation states also play an important.
For an ionic compound. Chemical formulas represents one formula unit. F-Fluoride N3-Nitride Aluminum cation Br-_____Bromide.
PREDICTING IONIC CHARGES Oxidation numbers. The ions charges that atoms gain when they lose or gain their valence electrons. Are the number of electrons they can lose or gain when bonding.
PREDICTING IONIC CHARGES Group 1 Lose 1 electron to form 1 Group 2. Chemical Structure and Reactivity. A degree level book.
Introducing inorganic organic and physical chemistry. First year undergraduate book. These books are definitely not popular science books for a little light reading.
The first two books are on the reading list for pre- Natural Sciences at Cambridge. If you are thinking about applying to do Natural. Yes its possible to remove fluoride from water.
Its a difficult water treatment action but there are several methods that can remove the fluoride ion although they may vary in their effectiveness. To truly get non-fluoridated water the fluoride ions have to be tackled at the molecular or chemical level to ensure effective elimination. Corrosion is a dissolving or wearing away of metal caused by a chemical reaction between water and your plumbing.
A number of factors are involved in the extent to which lead enters the water including. The chemistry of the water acidity and alkalinity and the types and amounts of minerals in the water the amount of lead it comes into contact with the temperature of the water the amount. Calcium sulfate 3 C 2 Br 6 dicarbon hexabromide 4 CrCO 3 3 chromium VI carbonate 5 Ag 3 P silver phosphide 6 IO 2 iodine dioxide 7 VO 2 vanadium IV oxide 8 PbS lead II sulfide 9 CH 4 methane 10 N 2 O 3 dinitrogen trioxide Write the formulas of the following chemical compounds.
11 tetraphosphorus triselenide P 4 Se 3 12. The compound forms a calcium fluoride-like material on the enamels surface that releases fluoride for remineralization when the pH in the mouth drops. Thus professional tooth cleaning solely to prepare the teeth for application of a fluoride compound is unnecessary.
Toothbrushing and flossing appear equally effective in improving the efficacy of high-concentration fluoride compounds 231.