
Chemical formulas can be. C Barium chloride sulphur dioxide and water are formed when dilute hydrochloric acid is added to this solution of barium sulphate and sodium chloride.
They are each made of a ca.
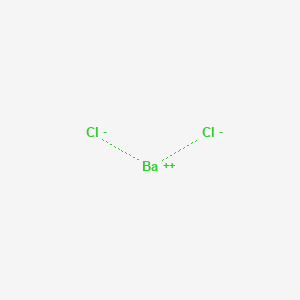
Chemical formula for barium chloride. The chemical formula of sucrose is C12H22O11 meaning that each molecule of sucrose has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms. The chemical formula for table salt otherwise known as sodium chloride is NaCl. Barium chloride is an inorganic compound with the formula Ba Cl 2.
It is one of the most common water-soluble salts of barium. Like most other barium salts it is white toxic and imparts a yellow-green coloration to a flame. It is also hygroscopic converting first to the dihydrate BaCl 2 H 2 O 2.
It has limited use in the laboratory and. Barium chloride BaCl2 CID 25204 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. An ionic compound is composed of a metal and a non-metal. Sodium chloride NaCl and magnesium oxide MgO.
The transfer of electrons between metals and non-metals produces charged particles called ions. Metals lose electrons to produce positve ions called cations. Na Mg 2 Non.
Chemical formula plays an important role in understanding different concepts of chemistry. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
Chemical formulas can be. Since both barium chloride and barium sulfate contain one mole of barium atoms pert mole of compound the moles of barium sulfate will be the same 0100 when barium has the limiting. Formula of barium nitrate.
Construct the chemical formula of ironII chloride. There is an easier way to determine the number of cations and anions. You only need to interchange the value of the charges of the ions as shown in the following examples.
However when the cation and anion have the same value of charges you need to determine the simplest ratio of the ions. A chemical formula is a shorthand method of representing the elements in a compound. The formula shows the formulas of the elements in the compound and the ratio of the elements to one another.
For example the forumla for sodium chloride. NaCl tells us that the compound is composed of the elements sodium Na and chlorine Cl in a one-to-one ratio. That is one atom of sodium combines with.
Answer 1 of 5. When you get questions like this it helps a lot if you can see the general sorts of patterns. I will give the lowest level of explanation and then follow up with a high level explanation.
In this case both of the reactants are salts. They are each made of a ca. 10361-37-2 BaCl 2 2H 2 O.
13718-50-8 BaI 2 2H 2 O. A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound. Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O.
Similarly sulfuric acid has a chemical formula H₂SO₄ which means that in this compound the. Barium is a naturally occurring alkaline metalloid element with atomic symbol Ba atomic number 56 and atomic weight 137 that is only found in combination with other elements typically barite barium sulfate and witherite barium carbonate or chemicals. Barium is used in many industrial processes as well as in diagnostic testing fireworks and pesticides.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character.
Fe Au Co Br C O N F. Ionic charges are not yet supported and will be ignored. BaOH2 barium hydroxide 16.
PbCl2 lead II chloride 7. Na2S sodium sulfide 17. KNO3 potassium nitrate 8.
Iron II chloride 18. MgOH2 magnesium hydroxide 9. Na2CrO4 sodium chromate 19.
LiCIO3 lithium chlorate 10. NH42SO4 ammonium sulfate 20. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
GoldIII Chloride B2H6 Diborane B2O3 Boron Oxide B5H9 Pentaborane BaNO32 Barium Nitrate BaOH2 Barium Hydroxide BaCl2 Barium Chloride BaCO3 Barium Carbonate BaI2 Barium Iodide BaO Barium Oxide BaO2 Barium Peroxide BaSO4 Barium Sulfate BBr3 Boron Tribromide BCl3 Boron Trichloride BeCl2 Beryllium Chloride BeI2 Beryllium Iodide BF3 Boron. For example barium chloride reacts with sodium sulphate and forms sodium chloride and precipitate of barium sulfate. Reaction BaCl 2 Na 2 SO 4 BaSO 4 NaCl.
Change in State Some chemical reactions are accompanied by change in state. For example ammonia gas reacts with hydrogen chloride gas and forms solid. Chemical Formulae and Equation Calculation.
Molar volume. Molar volume. Molar mass.
Number of particles. Mole of particles. Mass of particle in gram molar mass.
Avogadro Constant. Volume of Gas. For Solid liquid or gas.
Mass of subtance number of mole molar mass. Molar mass RAMRMMRFM in gram. For gas only volume of gas.
Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. C 4 H 10 O. C 4 H 8 O 2.
CdNO 3 2. C Barium chloride sulphur dioxide and water are formed when dilute hydrochloric acid is added to this solution of barium sulphate and sodium chloride. Since barium chloride is a soluble substance thus white precipitate of barium sulphite disappears.
BaSO 3 s HCl ag BaCl 2 ag SO 2 g H 2 O. Chemical Formula Chemical Formula Aluminum Oxide Benzene Latex Charcoal Ammonia Ink Boron Trioxide Lithium Hydride Crude Oil Barium Sulfate Iron Sulfide Calcium Bromide Luminol Lye Glue Cyanoacrylate Hydrogen Peroxide Al2O3 C6H6 C5H8 C7H4O NH3 FeSO4 B2O3 LiH C9H20 BaSO4 FeS CaBr2 C8H7N3O2 NaOH C5H5NO2 H2O2 CRAFTABLE COMPOUNDS Here are the compounds you can.