
There are also symbols in chemical equations for groups of chemical elements for example in comparative formulas. Chemical reactions balanced and unbalanced chemical equations.
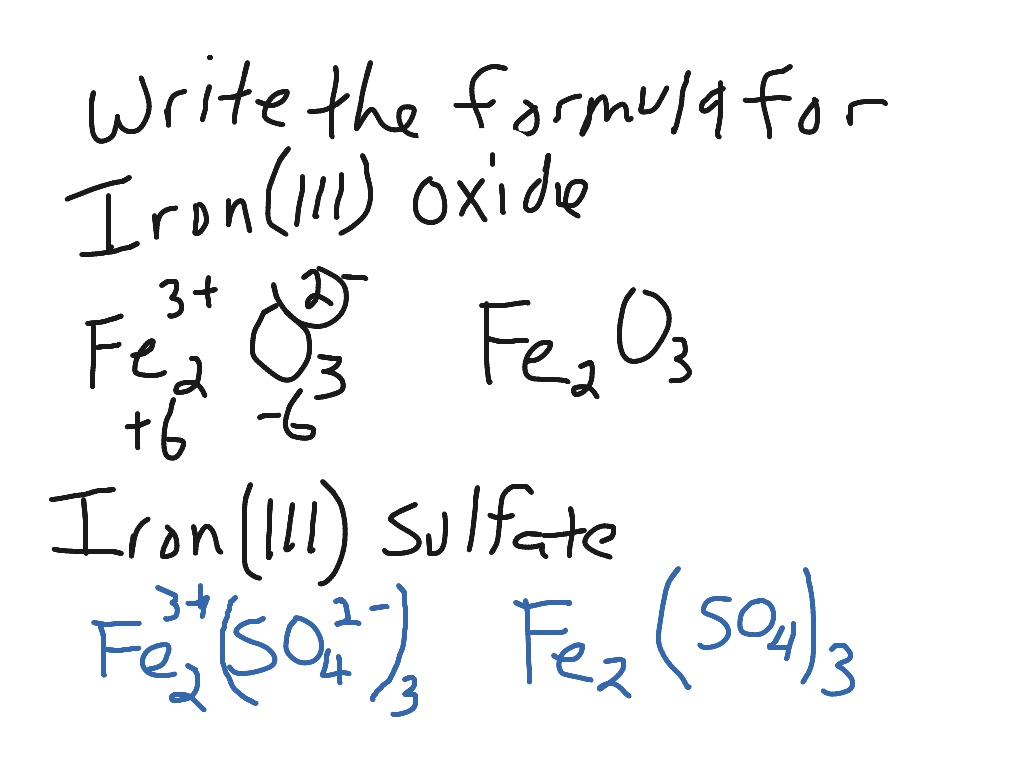
Predict the solubility of common inorganic compounds by using solubility rules.
Chemical equations for iron in body. Chemical Reactions -A chemical reaction is in which the bonds are broken within reactant molecules and new bonds are formed within product molecules in order to form a new substance. Chemical reactions are all around us. Chemical reactions are continually taking place on our planet.
To learn Definition Equations Types Examples with FAQs of Chemical Reactions. Chemical reactions and equations 2. This glucose combines with oxygen in the cells of our body and provides energy.
This is an endothermic reaction too. Decomposition reactions When a product breaks up into its constituent reactants the reaction is termed as decomposition reaction. 2FeSO4 s ___Heat___Fe2O3s SO2 g SO3 g In this reaction you can observe that a single.
Chemical Reactions and Equations CHAPTER1 Consider the following situations of daily life and think what happens when n milk is left at room temperature during summers. N an iron tawapannail is left exposed to humid atmosphere. N grapes get fermented.
N food is cooked. N food gets digested in our body. In all the above situations the nature and the identity of the initial.
Iron Heavy Metal. Iron is a chemical element in the periodic table that has the symbol Fe and atomic number 26. Iron is a group 6 and period 4 metal.
Iron is notable for being the final element produced by stellar nucleosynthesis and thus the heaviest element which does not require a supernova or similarly cataclysmic event for its formation. It is therefore the most abundant heavy. Chemical reactions balanced and unbalanced chemical equations.
May 10 2018 Sushil Humagain Chemistry 0. Temporary. Melting of ice or wax magnetizing or demagnetizing of iron preparation of solution making of different objects from wood soil paper etc.
NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations includes all the important topics with detailed explanation that aims to help students to understand the concepts better. Students who are preparing for their Class 10 exams must go through NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations. Check CBSE Class 10 Science Chapter 1 - Chemical Reactions and Equations MCQs to prepare for the objective type questions in Board Exam 2021-02022.
Digestion of food in the body. Dissolution of sugar in water. MCQ Questions for Class 10 Chemical Reactions and Equations Question No 38.
Which one of the following processes involve chemical reactions. Storing of oxygen gas under pressure in a gas cylinder. Keeping petrol in a.
Sometimes you find it challenging to understand the chemical symbols and chemical equations. Chapter 1 of science is full of theories and different types of chemical equations and reactions which makes it confusing for you to understand the chapter without proper notes and study material. The equations are sometimes difficult to understand and bars you from securing good marks in the exams.
Rusting of iron. Name and state the law which is kept in mind while we balance a chemical equation. Law of conservation of mass.
Mass can neither be created nor be destroyed during a chemical reaction. State one basic difference between a physical change and a chemical change. Chemical equations define how specific chemicals interact and react with one another.
For simple reactions the chemical equation is a single process however many complex reactions occur that require the combining of multiple equations into a final equations that takes into account all the reactants and products. You combine multiple reactions into a single equation by listing all the. Balanced chemical equation The chemical equation that shows the chemical reaction needs to be balanced.
A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side. Zn H 2 SO 4 ZnSO 4 H 2 3Fe s 4H 2 O g Fe 3 O 4 s 4H 2 g. Iron 26 Fe 55845.
Cobalt 27 Co. There are also symbols in chemical equations for groups of chemical elements for example in comparative formulas. These are often a single capital letter and the letters are reserved and not used for names of specific elements.
For example an X indicates a variable group usually a halogen in a class of compounds while R is a radical meaning a. Common Chemical Changes. The rusting of iron.
Combustion burning of wood. The metabolism of food in the body. Mixing an acid and a base such as hydrochloric acid HCl and sodium hydroxide NaOH.
Smelting is a process of applying heat to ore in order to extract a base metal. It is a form of extractive metallurgyIt is used to extract many metals from their ores including silver iron copper and other base metalsSmelting uses heat and a chemical reducing agent to decompose the ore driving off other elements as gases or slag and leaving the metal base behind. This chemical reaction is hydrated copper carbonate and a famous example of it is the Statue of Liberty.
Built in 1886 the Statue of Liberty was initially reddish-brown. Over time its copper plates underwent a chemical reaction. The same things can happen to copper pennies.
A similar reaction occurs when iron rusts. Iron oxide forms on its surface oxidation causing the iron to turn a. Powered by FlexBook textbook Platform CK-12 Foundation 2021.
Chemical changes on the other hand are quite different. A chemical change occurs when the substances composition is changed. When bonds are broken and new ones are formed a chemical change occurs.
The following are indicators of chemical changes. Noticeable Odor after reaction has begun Formation of a Precipitate. Note that in chemical equations and calculations that mole concentrations are abbreviated as mol.
First the needs of men and women for some minerals are different. The extreme case is for iron. Women need over twice as much as men do.
In all other cases where there is a different RDI men need more than women. Second the amounts of the various minerals needed on a daily basis vary widely. Write word equations for two chemical reactions with the help of materials given in the box.
Air copper sulphate iron vinegar iron oxide carbon dioxide iron sulphate copper lime water water. I Iron air water iron oxide ii Copper sulphate iron iron sulphate copper. Physical and Chemical Changes Class 7 Science Extra Questions Short Answer Type Questions.
The Iron-Thiocyanate Equilibrium When potassium. Equations reactions tables and diagrams can be written by hand. For this first lab report we will write the Introduction and Procedure sections for you.
Note the style of these sections you will be required to write your own Introduction and Procedure for a Subsequent Report see the Report FormThe sections of your. Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Identify common acids and bases.
Predict the solubility of common inorganic compounds by using solubility rules. Compute the oxidation states for elements in compounds. Humans interact with one another in various and complex ways and we classify these interactions according to common.
Chemical formulas are a way of representing the elements in a compound. Chemical formulas contain atomic symbols and digits to show the ratio of. Iron Coffin Examination of Nicolo Dettis Body.
Upon investigating one of the more harrowing deaths in the game Sherlock will notice the out of place foaming around the corpses mouth. Chemical shifts result from diamagnetic susceptibility effects at the atomicmolecular level. Susceptibility is a response of matter to an external magnetic field that may arise from several different mechanisms described in a prior QAThe easiest way to think about the phenomenon is to imagine that the electrons circulating around the nucleus produce an induced magnetic field Bind that.