Energy of third ionisation. Look over the flow chart and notes from today to review the concepts.
Again it introduces a new concept radioactive decay.
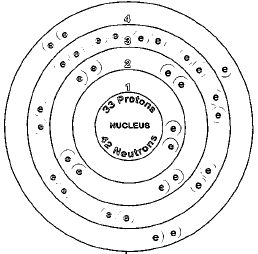
Chart of all arsenic isotopes. Thus different isotopes of a given element all have the same number of electrons and share a similar electronic structure. Because the chemical behavior of an atom is largely determined by its electronic structure different isotopes exhibit nearly identical chemical behavior. The main exception to this is the kinetic isotope effect.
Due to their larger masses heavier isotopes tend to react. Electronic shell Ar 3d 10 4s 2 4p 3. Energy of first ionisation.
Energy of second ionisation. Energy of third ionisation. Standard potential - 03 V As 3 As Discovered by.
Arsenic appears in three allotropic forms. Yellow black and grey. The stable form is a silver-gray brittle crystalline solid.
Uranium is weakly radioactive because all isotopes of uranium are unstable with half-lives varying between 159200 years and 45 billion years. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70 higher than that of lead and slightly lower than that of gold or tungsten.
Stable Isotopes Chart - Stable Isotopes of all the elements in table chart. This Stable Isotopes chart table gives the Stable Isotopes of all the elements of periodic table. Click on Element Atomic Number Element Symbol Element Name and Element Stable Isotopes headers to sort.
Element Atomic Number Element Symbol Element Name Element Element Stable Isotopes 1. Electronis shell Ne 3s 2 3p 6. Energy of first ionisation.
Energy of second ionisation. Energy of third ionisation. Sir Ramsay in 1894.
Argon was suspected to be present in air by Henry Cavendish in 1785 but wasnt discovered until 1894 by Lord Rayleigh and Sir William Ramsay. Argon is the third noble gas in. Of the first 82 elements in the periodic table 80 have isotopes considered to be stable.
The 83rd element bismuth was traditionally regarded as having the heaviest stable isotope bismuth-209 but in 2003 researchers in Orsay France measured the half-life of 209 Bi to be 19 10 19 years. Technetium and promethium atomic numbers 43 and 61 respectively and all the elements with an. Isotopes All Known Symbol.
Isotopes Stable Thermal Conductivity. Van Der Waals Radius. Fill in the isotope names and any missing information on the chart.
Use your periodic table and the information provided. Assume all atoms are neutral. Of protons 25 of neutrons 17 15 of electrons of protons 32 of neutrons 30 32 of electrons of protons of neutrons 48 51 of electrons 46 of protons.
Isotopes All Known Symbol. Isotopes Stable Thermal Conductivity. Van Der Waals Radius.
Periodic table of the elements materials science and academic information elements and advanced materials data scientific presentations and all pages designs concepts logos and color schemes herein are the copyrighted proprietary rights and intellectual property of American Elements. American Elements is a US. Quantum Numbers Chart - Quantum Numbers of all the elements in table chart.
This Quantum Numbers chart table gives the Quantum Numbers of all the elements of periodic table. Click on Element Atomic Number Element Symbol Element Name and Element Quantum Numbers headers to sort. 33 As Arsenic 74922.
34 Se Selenium 78971. 35 Br Bromine 79904. 36 Kr Krypton 83798.
37 Rb Rubidium 85468. 38 Sr Strontium 8762. 39 Y Yttrium 88906.
40 Zr Zirconium 91224. 41 Nb Niobium 92906. 42 Mo Molybdenum 9595.
43 Tc Technetium 98 44 Ru Ruthenium 10107. 45 Rh Rhodium 10291. 46 Pd Palladium 10642.
47 Ag Silver 10787. 48 Cd Cadmium 11241. 49 In Indium 11482.
These elements all line up in the eighteenth or last column of the periodic table. They all have a full outer shell of electrons making them very stable they tend not to react with other elements. Another example is the alkali metals which all align on the left-most column.
They are all very similar in that they have only 1 electron in their outer shell and are very reactive. You can see. Look over the flow chart and notes from today to review the concepts.
Read more about isotopes. Again it introduces a new concept radioactive decay. Just be aware that some isotopes are unstable and give off protons making a totally new element with another element or particle released.
Remember that when the atomic number changes it becomes an entirely new element.