Is nf3 polar. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition.
The product is a nitroso phenol O N OH HNO2 H2SO4.
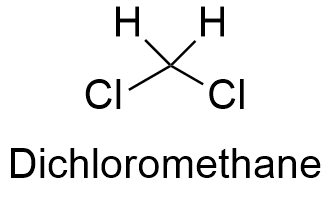
Ch2cl2 molar mass. C H 2 Cl 2. 8493 gmol 1. Appearance Colorless liquid Odor.
13266 gcm 3 20 C. 967 C 1421 F. Molar Mass of Frequently Calculated Chemicals.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
1 mole of this substance contains 1201 G of carbon 2016 G of hydrogen and 7090 G of chlorine. 1 mole of this substance contains 1 mol of carbon and 4 moles of chlorine. Correctly order the steps necessary to solve for the mass of a product or 2nd reactant required given the mass of one of the reactant in a chemical process.
Start with the first step at the top of the list. CH2Cl2 C2H2Cl2 C2H3Cl CCl4. The rate of diffusion of a gas is inversely proportional to its molar mass.
Assuming ideal behavior what is the density of argon gas at STP in gL. Multiple Choice 00176 gL 00250 gL 00561 gL 178 gL 181. What is the density of carbon dioxide gas at -252C and 980 kPa.
Multiple Choice 0232 gL 0279 gL 0994 g. Abbreviations and acronyms of chemical compounds EniG. Periodic Table of the ElementsKTF-Split 22 Jan.
How many moles of CH2Cl2 contain 7224 moles of chlorine. The smallest sample of carbon atoms that can be observed with the naked eye has a mass of approximately 2 times 10-8 g. Mole concept One mole is the amount of a substance that contains as many particles or entities as there are atoms in exactly 12 g of the 12C isotope.
1 mol of atom 60221023 entities. The mass of one mole of a substance in grams is called its molar mass. Assertion Reason Solid State QNo1 Which of the following is not a characteristic property of Solids.
A Intermolecular distances are short b Intermolecular forces are weak c Constituent particles have fixed positions d Solids oscillate about their mean positions Ansb. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition. Reactions of Alcohols OH PCC CH2Cl2 Na2Cr207 H2SO4 H2O Cr03 H2SO4 H2O PCC CH2Cl2 OH Cr03 H2SO4 H2O 0 Na2Cr2O7 H2SO4 H2O PCC CH2Cl2 16.
The alcohol and chromic acid form a chromate ester that either reacts intramolecularly or intermolecularly in the presence of a base water to yield the corresponding carbonyl compound. The product is a nitroso phenol O N OH HNO2 H2SO4. If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3.
According to the VSEPR theory the CHCl3 molecule possesses tetrahedral molecular geometry. Thus total seven compounds have non zero dipole moment. State Config State description.
Molar mass M is the mass in grams of one mole of a chemical substance. The molar mass of an element or compound is given the unit grams per mole or g mol1. For example the molar mass of carbon is 12 g mol1.
Therefore the molar mass of an element or compound is the relative atomic molecular or formula mass amount expressed in grams per mole. Examples Molar mass of CO2 12. Stereoisomers are very important in the chemistry biochemistry and medical fields.
Learn the definition of a stereoisomer and types and see examples like a geometric isomer or those with an. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition.
The molar mass of X is 77. The other molar masses are as follows. Therefore this molecule is nonpolar.
90 degrees is the bond angle because O is in group 6 and Oct 26 2021 SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. Lewis Structure H -cö-H 3. Solution for Determine whether moleculeion is polar.
Predict the major product of the following reaction. Is nf3 polar. D If the stoichiometry of the products is one metal per two bromine atoms the ratio of product to metal mass should be 7.
_4 forms ethene as the major product. What is the role played by concentrated H_2SO_4 in this reaction. Also write the chemical equation involved.
2 g of H2O to react. Predict the two major organic products of the following reaction. Chcl3 has how many double bonds.
C2br2 geometry - afiuniquspl. The cover illustrates an expedient one-step synthesis of α-ketoacetals via electrophilic etherification of α-alkoxy enolates and monoperoxyacetals. The chemistry of α-ketoacetal compounds is incredibly versatile.
α-Ketoacetals are able to be selectively transformed into α-hydroxy acetals which can undergo hydrolysis to form α-hydroxy acetals highly useful functional groups. The main fraction LF2 consisted of L-fucose D-galactose and sulfate at a molar ratio 619. A structural study on the LF2 was carried out by NMR spectroscopy.
The backbone of LF2 was primarily 1 3-linked α-L-fucopyranose residues 75 and a few 1 4-α-L-fucopyranose linkages 25. The branch points were at C-4 of 3-linked α-L-fucopyranose residues by β-D-galactopyranose.