
Three hydroxide ions would be needed to balance the charge on one iron ion. For example ironIII hydroxide indicates that the iron ion has a 3 charge.
Thus in most problems that arise pH values lie mostly in the range 0 to 14 though negative pH values and values above 14 are entirely possible.
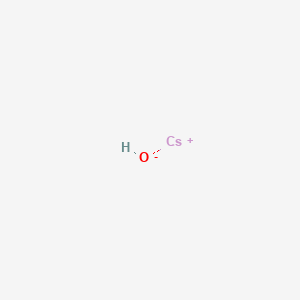
Cesium hydroxide formula. Cesium hydroxide should not be stored in glass containers. Containers of cesium hydroxide should be protected from physical damage and should be stored separately from acids organic compounds metals oxygen carbon dioxide moisture heat sparks and open flame. Because container that formerly contained cesium hydroxide may still hold.
Ionic Compounds Naming and Formula Writing. Copy this to my account. E-mail to a friend.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Ionic Compounds Naming and Formula Writing. Copy this to my account.
E-mail to a friend. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Polyatomic ions are ions which consist of more than one atom.
For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. PH solution acid base Chemistry hydrogen hydroxide ion pH solute solution PLANETCALC pH of a strong acidbase solution Timur 2020-11-03 141940. Rubidium hydroxide RbOH cesium hydroxide CsOH.
A solution of a strong acid at concentration 1 M 1 molL has a pH of 0. A solution of a strong alkali at concentration 1 M 1 molL has a pH of 14. Thus in most problems that arise pH values lie mostly in the range 0 to 14 though negative pH values and values above 14 are entirely possible.
Weak acidsbases only. Note that while calcium hydroxide barium hydroxide and strontium hydroxide are strong bases they are not very soluble in water. The small amount of compound that dissolves dissociates into ions but most of the compound remains a solid.
Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice. You should complete this by Sunday.
Use the stock form for the transition metals. Give the formula for the following. Sulfur dioxide SO2_ 2.
Sodium thiosulfate Na2S2O3_ 3. Ammonia solution also known as ammonia water ammonium hydroxide ammoniacal liquor ammonia liquor aqua ammonia aqueous ammonia or inaccurately ammonia is a solution of ammonia in water. It can be denoted by the symbols NH 3 aq.
Although the name ammonium hydroxide suggests an alkali with composition NH 4 OH it is actually impossible to isolate samples of NH 4 OH. SnOH2 tin II hydroxide 28. Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1.
Lead II chlorate PbClO32 2. Strontium acetate SrC2H3O22 3. Zinc phosphate Zn3PO42 4.
Ammonium cyanide NH4CN 5. Sodium carbonate Na2CO3 6. Lead IV dichromate PbCr2O72 7.
Copper I sulfite Cu2SO3 8. Cadmium phosphate Cd3PO42 9. Tin II bicarbonate.
For the transition metals the charge is indicated in the name with a Roman numeral in parentheses. For example ironIII hydroxide indicates that the iron ion has a 3 charge. Hydroxide is a polyatomic ion that has a -1 charge.
Three hydroxide ions would be needed to balance the charge on one iron ion. This yields the formula FeOH₃. Name Formula Charge Name Formula Charge Name Formula Charge.
Cesium Cs 1 potassium K 1 cyanide CN 1 chromium II Cr 2 2 rubidium Rb 1 dichromate Cr 2 O 7 2 2 chromium III Cr 3 3 scandium III Sc 3 3 dihydrogen phosphate H 2 PO 4 1 cobalt II cobaltous Co 2 2 silver Ag 1 fluoride F 1 cobalt III cobaltic Co 3 3 sodium Na 1 hydroxide OH. Caesium carbonate or cesium carbonate is a white crystalline solid compound. Caesium carbonate has a high solubility in polar solvents such as water alcohol and DMFIts solubility is higher in organic solvents compared to other carbonates like potassium and sodium carbonates although it remains quite insoluble in other organic solvents such as toluene p-xylene and chlorobenzene.
All soluble hydroxides like lithium cesium sodium potassium etc. An alkali is said to be strongest when it produces almost all OH ions when it is dissolved in water. As NaOH molecule when dissolved in water produce almost all OH ions that ultimately make it strong alkali.
Uses of Sodium hydroxide. Praseodymium hydroxide PrOH 3 339 1024 Compound Formula K sp Radium iodate RaIO 3 2 116 109 Radium sulfate RaSO 4 366 1011 Rubidium perchlorate RbClO 4 300 103 Scandium fluoride ScF 3 581 1024 Scandium hydroxide ScOH 3 222 1031 SilverI acetate AgCH 3 COO 194 103 SilverI arsenate. Simple Binary Ionic Compounds Please complete the following table.
Formula of Ionic Compound s 2. Name of Ionic Compound. Print Week 4 - Chemistry flashcards and study them anytime anywhere.
CsOH - cesium hydroxide CaOH 2 - calcium hydroxide SrOH 2 - strontium hydroxide BaOH 2 - barium hydroxide These bases completely dissociate in solutions of 001 M or less. The other bases make solutions of 10 M and are 100 dissociated at that concentration. There are other strong bases than those listed but they are not often encountered.
Properties of the Strong Bases. 632 Write the formula including the charge for each of the following polyatomic ions. A compound that consists of positive and negative ions derived from one or more electrons from metals being transferred to non metals.
What are ionic bonds. Formed when one or more electrons are transferred from. Formula Name NaOHaq sodium hydroxide KOHaq potassium hydroxide CaOH 2 aq calcium hydroxide NH 3 aq aqueous ammonia Approximate Indicator pH Range Color for Color Change Change methyl orange 3144 red to yellow bromthymol blue 6076 yellow to blue phenolphthalein 89 colorless to pink litmus 4583 red to blue bromcresol green 3854 yellow to blue thymol blue 809.
Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Cesium phosphide Cs3P calcium iodide CaI2 barium fluoride BaF2. II hydroxide CrOH2____ barium permanganate BaMnO42 mercuryII cyanide HgCN2 nickelII hydroxide NiOH2 magnesium bicarbonate ___MgHCO32 potassium dichromate K2Cr2O7 aluminum hydrogen sulfate AlHSO43 or bisulfate.
4 MOLECULAR COMPOUNDS When non-metal atoms share electrons with other non-metal. 2CO 3 calcium hydroxide CaOH 2 iron II fluoride FeF 2 lead IV cyanide PbCN 4 hypoiodous acid HIO acetic acid HCH 3COO arsenic V acetate AsCH 3COO 5 zinc carbonate ZnCO 3 lead II oxalate PbC 2O 4 oxalic acid H 2C 2O 4 periodic acid HIO 4 antimony III thiosulfate Sb 2S 2O 3 3 cesium carbide Cs 4C ammonium perbromate NH 4BrO 4. Hydroxide polyatomic anion.
For a cation is shown by a number and plus sign after the formula. If theres just a plus sign it means the charge is plus 1. Review some examples of cations or positive ions.
Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2 calcium Ca 2 cesium Cs chromium III Cr 3 cobalt Co 2 copper I Cu copper II Cu 2 hydrogen H iron II Fe 2 iron. Mg 2 magnesium ion. Ca 2 calcium ion.
Sr 2 strontium ion. Ba 2 barium ion. Al 3 aluminum ion.
Transition B-group and Post-Transition Group IVA and VA Metals. These elements usually form ionic compounds. Many of them can form more than one cation.
The charges of the common transition metals must be memorized. Group IV and V metal cations.