006 mg bariumanimal as barium carbonate was given im to rats strain not given. 8 H 2 O sulfate double de cadmium et dammonium sulfate double de.

Nitrites react with potassium iodide in the presence of dilute sulphuric acid to liberate iodine.
Carbonate de cadmium. The colorfastness or permanence of cadmium requires protection from the elements tendency to slowly form carbonate salts with exposure to air. Most paint vehicles accomplish this but cadmium colors will fade in fresco or mural painting. The following are commonly used as pigments in artists paints.
Cadmium yellow is cadmium sulfide CdS CI. Cadmium sulfoselenide is a. The chemical element Calcium Ca atomic number 20 is the fifth element and the third most abundant metal in the earths crust.
The metal is trimorphic harder than sodium but softer than aluminiumA well as beryllium and aluminium and unlike the alkaline metals it doesnt cause skin-burns. It is less chemically reactive than alkaline metals and than the other alkaline-earth. It was discovered in 1817 simultaneously by Stromeyer and Hermann both in Germany as an impurity in zinc carbonate.
Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel and cadmium compounds are used as red orange and yellow pigments to color glass and to stabilize plastic. 006 mg bariumanimal as barium carbonate was given im to rats strain not given.
Most of the dose was resorbed from the injection site within 3 days. 20 of the initial dose remained in the body mostly in the skeleton after 280 days. Average barium carbonate concentration at 1.
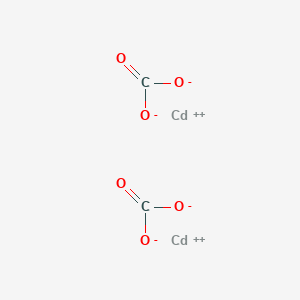
Carbonate de cadmium CdCO 3 pK s 113. Corps naturel nommé otavite iodates. Iodate de cadmium CdIO 3 2 pK s 76 sulfates.
Sulfate de cadmium CdSO 4 orthorhombique sulfate de cadmium hydraté CdSO 4. 4 H 2 O monoclinique sulfate de cadmium hydraté CdSO 4. 7 H 2 O monoclinique sulfate de cadmium hydraté 3 CdSO 4.
8 H 2 O sulfate double de cadmium et dammonium sulfate double de. IronII hydroxide ferrous hy. IronIII hydroxide ferric hy.
K 2 CO 3. K 2 CrO 4. K 2 HPO 4.
Cadmium arsenate Cd 3 AsO 4 2 22 1033 Cadmium carbonate CdCO 3 10 1012 Cadmium fluoride CdF 2 644 103 Cadmium hydroxide CdOH 2 72 1015 Cadmium iodate CdIO 3 2 25 108 Cadmium oxalate trihydrate CdC 2 O 4 3H 2 O 142 108 Cadmium phosphate Cd 3 PO 4 2 253 1033 Calcium carbonate. The color fastness or permanence of cadmium requires protection from its tendency to form carbonate salts when exposed to air. Nowadays cadmium dyes have been partially replaced by azo dyes.
Low levels exposure to Cd may lead to damage to the kidneys liver skeletal system and cardiovascular system as well as to deterioration of sight and hearing. Along with strong teratogenic. Sulphides react with a suspension of cadmium carbonate to form a yellow precipitate of cadmium sulphide.
Confirmation of Nitrite NO 2- a Ferrous sulphate test. Nitrites give a dark brown or black colouration in Ferrous sulphate test due to the formation of FeSO 4NO. B Starch - Iodide test.
Nitrites react with potassium iodide in the presence of dilute sulphuric acid to liberate iodine. PH of Common Acids and Bases. Calculated pH values of common acids and bases for 1 10 and 100 mmolL valid for standard conditions at 25 1 atm.
Acidity constants are taken from here. Cadmium chloride is a white crystalline solid. It is soluble in water.
The primary hazard of this material is that it poses a threat to the environment. Immediate steps should be taken to limit its spread to the environment. Cadmium chloride is used in photography in fabric printing in chemical analysis and in many.
Lintoxication au cadmium peut se faire de manière aiguë ou chronique avec des lésions essentiellement pulmonaires osseuses et rénales. Le cadmium na pas de rôle physiologique dans le corps humainLe métal lui-même et ses composés sont extrêmement toxiques même à faibles concentrations et ont tendance à saccumuler dans les organismes vivants et les écosystèmes. Which evaluates contaminants such as lead cadmium nitrate and nitrite in addition to food additives.
Further up-to-date information on the GDWQ and the process of their development is available on the WHO Internet site and in the current edition of the GDWQ. Acknowledgements The first draft of Hardness in Drinking-water Background document for development of WHO Guidelines for Drinking. Residues obtained by cementation of cadmium by iron dust out of cadmium sulfate solutions.
Composed primarily of metallic cadmium and zinc. Composed primarily of metallic cadmium and zinc. 293-309-7 CAS No.
Schwermetalle sind unter uneinheitlichen Definitionen zusammengefasste Metalle deren Dichte oder Atommasse einen bestimmten Wert übersteigt. Teilweise werden in die Definition noch weitere Eigenschaften wie Ordnungszahl und Toxizität einbezogen. Viele Quellen stufen als Schwermetall ein Metall ein dessen Dichte größer als 50 gcm³ oder bei älteren Quellen größer als 45 g.
Cadmium sulfate icsc titanium carbide icsc tungsten carbide icsc attapulgite icsc radon icsc calcium fluoride icsc aluminium fluoride anhydrous icsc beryllium oxide icsc hydrogen iodide icsc chlorotoluron icsc paracetamol icsc benzyl acetate icsc 1-bromopropane icsc ammonium hydrogen carbonate icsc butyric acid icsc. Milliequivalent mEq mile-e-kwivah-lent one thousandth 103 of a chemical equivalent see equivalent weight. Concentrations of electrolytes are often expressed as milliequivalents per liter which is an expression of the chemical combining power of the electrolyte in a fluid.
Miller-Keane Encyclopedia and Dictionary of Medicine Nursing. Cadmium Cd is toxic to the kidneys can demineralise bones and is carcinogenic. Cd is present naturally in soils and is also added to soils by P fertilisers and atmospheric deposition.
Several factors including soil structure and soil chemistry humus content and pH affect the plant availability of Cd. The application of Cd-containing fertilisers increases Cd concentrations in the crops. The Color of Art Pigment Database - the Pigment Yellow page of the database is a complete artists pigment reference with color Index names pigment chemical composition lightfastness safety and other information on artists pigments and paint.
Indeed barium carbonate is useful as rat poison. Unlike barium sulfate barium carbonate dissolves in stomach acid releasing the poisonous barium to do its rather nasty but efficient work. Conveniently barium which is a soft silvery metallic alkaline earth metal is never found in nature in its pure form due to its reactivity with air or in water.
In fact the metal is a getter in vacuum. Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere. Bases Carbonate de sodium Sulfures de sodium Amas de copeaux Débris de démolition Dépôt de pâtes et papiers Matériaux secs.
Terres contaminées dhydrocarbures de Haute-Mauricie. Hydrocarbures pétroliers C10 à C50. Résidus de produits pétroliers.
Parc à résidus miniers Anacon Lead. Acides minéraux Métaux. It is the 5th most abundant element in the earths crust occurring widely as calcium carbonate which is more commonly known as limestone.
It is also the fifth most abundant dissolved ion in seawater. Calcium was named after the Latin term calx meaning lime and is a reactive silvery metallic element found in Group 2 of the periodic table. It was first isolated in 1808 in England when Sir.
Discovered in 1817 cadmium occurs in association with zinc ores. Almost all cadmium is a byproduct of processing ores for zinc copper and lead. Cadmium is a soft bluish-white metal that can be easily cut and is similar in behavior to zinc.
It is a component of low-melting alloys and used in electroplating solder standard EMF.