What is the atomic symbol for the noble gas that also has this electron configuration. Some of the worksheets displayed are Since we use different methods in naming binary covalent Formulas and nomenclature binary ionic Naming work 6 binary covalent compounds answer key Binary molecular compound practice Naming ionic compounds practice work Writing and naming binary compounds answers Naming formula writing for type 1.

Silicon can also be made into cermet composites which are resistant to high temperatures toughness and can be cut.
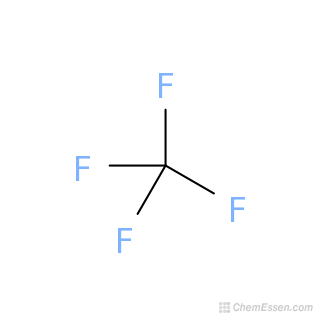
Carbon tetrafluoride symbol. Carbo coal is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. Carbon makes up only about 0025 percent of Earths crust.
Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a radionuclide. Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen.
Organic carbon compounds are far more numerous than inorganic carbon compoundsIn general bonds of carbon with other elements are covalent bondsCarbon is tetravalent but carbon free radicals and carbenes occur as short-lived. The carbon-nitrogen compounds are cyanogen CN 2 hydrogen cyanide HCN cyanogen chloride CNCl etc. The common carbon halides are carbon tetrafluoride CF 4 carbon tetrachloride CCl 4 carbon tetrabromide CBr 4 carbon tetraiodide C I 4 etc.
Uses of carbon and its compounds. Carbon is the basic building. 2 with carbon at high temperature.
SiO 2 s 3 C s SiC s 2 CO g How many grams of SiC can form when 300 g of SiO 2 and 450 g of carbon are reacted. Calculate the percent carbon hydrogen and oxygen in aspirin C 9 H 8 O 4. A compound is analyzed and found to contain 104 C 278 S and 617 Cl by mass.
P2s3 ionic or covalent. Write the chemical formula for carbon tetrafluoride. Write the chemical formula for krypton dichloride.
Write the chemical formula for tetraphosphorus hexasulfide. Compound A contains 157 g of sulfur and 186 g of fluorine. Compound B contains 254 g of sulfur and 602 g of fluorine.
A What is the mass ratio of fluorine to sulfur in Compound. High-purity monocrystalline silicon is an important semiconductor material that can be used as a solar cell to convert radiant energy into electrical energy which is a promising material in the development of energy. Silicon can also be made into cermet composites which are resistant to high temperatures toughness and can be cut.
Again xenon and fluorine gas are mixed in a 1. 2 volume ratio to produce xenon tetrafluoride at certain pressures and temperatures. Xe 2F XeF 4.
Properties of fluorineF atom. The atomic number of fluorine atoms is 9. The atomic number of an element is the number of electrons and protons in that element.
That is the number of electrons. We would like to show you a description here but the site wont allow us. What is the atomic symbol for the noble gas that also has this electron configuration.
How many oxygen atoms are present in each of the formulas. Classify each compound as ionic or molecular. NaBr CO2 CH4 ZnCl2.
NaBr and ZnCl2 Molecular. Write the name for the compound. The chemical symbol for the sodium ion is Na 1 or just Na.
Similarly if a chlorine atom gains an extra electron it becomes the chloride ion Cl. Both ions form because the ion is more stable than the atom due to the octet rule. Forming an Ionic Bond.
Once the oppositely charged ions form they are attracted by their positive and negative charges and form an ionic compound. Sicl4 intermolecular forces. The SiCl4 S i C l 4 is a non-polar molecule.
This effect illustrated for two H2 molecules in part b in Figure PageIndex3 tends to become more pronounced as atomic and molecular masses increase Table PageIndex2. Albr3 hybridization email protected email protected. The elements Name Symbol Atomic number Molar mass g mol1 Name Symbol Atomic number Molar mass g mol1 Actinium Alu Lewis structures 33 2.
N2O make sure to check formal charges III. Polar molecules have a high boiling point melting point low vapour pressure and high surface tension. 13 Draw Lewis Structures.
NO 2-1 SO Hybridization of. SiF 4 Silicon tetrafluoride g. CO 2 Carbon dioxide c.
Some of the worksheets displayed are Since we use different methods in naming binary covalent Formulas and nomenclature binary ionic Naming work 6 binary covalent compounds answer key Binary molecular compound practice Naming ionic compounds practice work Writing and naming binary compounds answers Naming formula writing for type 1. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition.
Your typical collection of carbon atoms is 989 carbon 12 with 6 neutrons and a mass of 12 u by definition 11 carbon 13 with 7 neutrons and a mass of 1300335 u measured and a minute but detectable trace of carbon 14 with 8 neutrons and a mass of 14003241 u measured. This averages out to 12011 u for carbon on the Earth taken from non-living or never alive sources. Methane and oxygen react to yield carbon dioxide and water in a 1212 ratio.
This ratio is satisfied if the numbers of these molecules are respectively 1-2-1-2 or 2-4-2-4 or 3-6-3-6 and so on Figure 2. Likewise these coefficients may be interpreted with regard to any amount number unit and so this equation may be correctly read in many ways including. Silicon is a chemical element with symbol Si and atomic number 14.
Classified as a metalloid Silicon is a solid at room. Gay Lussac and Thenard probably prepared impure amorphous silicon by heating potassium with silicon tetrafluoride. In 1824 Berzelius generally credited with the discovery prepared amorphous silicon by the same general method and purified the product by removing the.
The reaction between methane and oxygen to yield carbon dioxide and water shown at bottom may be represented by a chemical equation using formulas top. This example illustrates the fundamental aspects of any chemical equation. The substances undergoing reaction are called reactants and their formulas are placed on the left side of the equation.
The substances generated by the reaction are. Solution for For 125 mol of an ideal gas Pexternal P 350 x 103 pa. The temperature is changed from 135C to 212C and Cvm 3R2.
Calculate q w U. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
Silicon powder amorphous appears as a dark brown powder. Insoluble in water and denser than waterBurns readily when exposed to heat or flames and may be difficult to extinguish. Water may not be effective in extinguishing flames.
Used to make computer microchips. Sucrose is composed of the elements carbon hydrogen and oxygen. 02 x 1023 of anything usually atoms or molecules.
Dec 27 2017 Unit 7 Balancing Chemical Equations Worksheet 2 Answers Tessshlo. Write the color of the solid formed upon addition of KI to the supernatant. C4H10 O2 combustion 2C4H10 13O2 8CO2 10H2O 7.
If3 valence electrons.