Lets take a look at. Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen.

Capitalization and subscripts are graded.
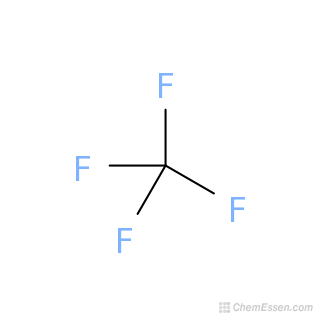
Carbon tetrafluoride chemical formula. Tetrafluoromethane also known as carbon tetrafluoride or R-14 is the simplest perfluorocarbon C F 4As its IUPAC name indicates tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methaneIt can also be classified as a haloalkane or halomethaneTetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the. The physical and chemical properties of carbon depend on the crystalline structure of the element.
Carbon forms compounds with the halogens with CX 4 as general formula where X is fluorine chlorine bromine or iodine. At ambient temperature carbon tetrafluoride is gas tetrachloride is liquid and the other two compounds are solids. We also know mixed carbon tetrahalides.
Carbo coal is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. Carbon makes up only about 0025 percent of Earths crust.
Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a radionuclide. Each chemical substance has a specific chemical composition so these chemical substances have their own chemical formula. Lets take a look at.
The chemical formula CF 4 represents Carbon Tetrafluoride. It is also known as a Tetrafluoromethane IUPAC name and R-14 owing to its use as a refrigerant. CF 4 is the simplest perfluorocarbon Hydrocarbons in which C-F bonds have replaced all the C-H bonds.
The compound is very stable and does not react with acids or hydroxides. The energy required to break the bonds in one mole of a chemical compound. Nonpolar covalent bond.
A covalent bond in the bonding electrons are equally attracted to both atoms. A covalent bond in which a shared pair of electrons is held more closely by one of the atoms. A molecule in which one end has a partial positive charge and the other end has a partial.
Click the Chemical Formula to see the Lewis Structure. Acetone C 3 H 6 O AsCl 3 Arsenic Trichloride AsF 3 Arsenic Trifluoride AsF 5 Arsenic Pentafluoride AsF 6-AsF 6- AsH 3 Arsenic Trihydride AsO 3 3-Arsenite Ion BBr 3 Boron Tribromide BCl 3 Boron Trichloride BF 3 Boron Trichloride BF 4-Tetrafluoroborate Ion BH 3 Boron Hydride BH 4-BH 4- BOH 3 BOH 3 BeCl 2. The chemical formulas for covalent compounds are referred to as molecular formulas A chemical formula for a covalent compound.
Because these compounds exist as separate discrete molecules. Typically a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table except that hydrogen is almost never written first H 2 O is the prominent exception. CHEMICAL GAS NAME FORMULA COLUMN A COLUMN B Acetylene Ethyne C2H2 137 437 Air 08N2-02O2 122 889 Allene C3H4 88 _____ Ammonia NH3 209 1188 Argon Ar.
Carbon Tetrabromide CCl2F2 Dichlorodifluoromethane CCl4 Carbon Tetrachloride CdNO32 Cadmium Nitrate CdS Cadmium Sulfide CF4 Carbon Tetrafluoride CH2Cl2 Dichloromethane CH2O Formaldehyde CH2O2 Formic Acid CH3COOH Acetic Acid CH4 Methane CH4O Methanol CHCl3 Chloroform Cl2 Chlorine Gas ClO2 Chlorine Dioxide CoNO32 CobaltII Nitrite CO2 Carbon. N 2 F 6. P 2 S 3.
P 2 O 5. Water is a liquid at room temperature. Carbon dioxide and carbon tetrafluoride are gases.
None of these compounds is composed of ions. A different attractive interaction between atoms called covalent bonding is involved here. Covalent bonding occurs by a sharing of valence electrons rather than an outright electron transfer.
Similarities in physical properties they are all gases suggest. Chemical Bond Questions and Answers. Get help with your Chemical bond homework.
Access the answers to hundreds of Chemical bond questions that are explained in a. 9 CO carbon monoxide 10 P 4 phosphorus 11 dinitrogen trioxide N 2 O 3 12 nitrogen N 2 13 methane CH 4 14 lithium acetate LiC 2 H 3 O 2 15 phosphorus trifluoride PF 3 16 vanadium V oxide V 2 O 5 17 aluminum hydroxide AlOH 3 18 zinc sulfide ZnS 19 silicon tetrafluoride SiF. Formula Cation Formula and name Anion Formula and name Compound Name Example.
NaCl Na sodium ion Cl- chloride ion Sodium chloride 1. CaBr2 2Ca calcium ion Br- bromide ion Calcium bromide 2. Mg 3N2 3-Mg 2 magnesium ion N nitride ion Magnesium nitride 3.
K2S K potassium ion S2- sulfide ion Potassium sulfide 4. ZnO Zn 2 zinc ion O2- oxide ion Zinc oxide 5. SnO 2 2-Sn 4.
9 CO carbon monoxide. For each of the following questions determine whether the compound is ionic or covalent and write the appropriate formula for it. 11 dinitrogen trioxide N2O3.
14 lithium acetate LiC2H3O2. 15 phosphorus trifluoride PF3. 16 vanadium V oxide V2O5.
This pictures shows examples of chemical bonding using Lewis dot notation. Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen. Bonds especially covalent bonds are often represented as lines between bonded atoms.
Acetylene has a triple bond a special type of covalent bond that will be discussed later. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical or physical change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule CH 4 and two diatomic oxygen molecules O 2 to produce one carbon dioxide molecule CO 2 and two water molecules H 2 O.
Chemical name Chemical formula Global warming potential. Tetrafluoromethane perfluoromethane carbon tetrafluoride CF 4. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical or physical change involves writing and balancing a chemical equation.
Consider as an example the reaction between one methane molecule CH 4 and two diatomic oxygen molecules O 2 to produce one carbon dioxide molecule CO 2 and two water molecules H 2 O. So now we have CH2O which is the dot structure for which is the molecular formula I should say for formaldehyde. And so if were following our guidelines here the first thing we need to do is find out the valence electrons.
So we need to look and find carbon hydrogen oxygen on our periodic table. So lets go back up here and lets find those elements here. Chemical Formula - Indicates the number and type of atoms in the base unit of a compound.
Type of compound Base unit Ionic Formula unit fu Molecular Molecule Valence Electrons - Electrons in the outermost shell of an atom The only e s involved in bonding and chemical reactions. For the S- P-blocks. Valence e Group number Ionic Compounds.
Common Chemical Name Formula Ethylene OxideCarbon Dioxide Mixtures C4 S S I I U I S S I I U U C3 UUUU Ethylene OxideHalocarbon Mixtures C4 S S I I U I S S I I U U C3 UUUU Ethylene OxideHCFC-124 C4 S S I I U I S S I I U U C3 UUUU Halocarbon 11 CCl3F SSSC5I SSSSSS U UC3SS U U Halocarbon 12 CCl2F2 SSSC5I SSSSSS U UC3SSSS Halocarbon 13 CClF3 SSSC5I SSSSSS U UC3SSSS Halocarbon. Write the formula for each compound. Nitrogen Monoxide Nitrogen Trifluoride.
Write the formula for each compound. Write the formula for each compound. Capitalization and subscripts are graded.
SeF4 OF2 N2O PCl3. Gas Facts includes charts and tables and interactive conversion formulas related to the chemical and physical properties of our cryogenic liquid and compressed gas products as well as an online tool for estimating the cost of using nitrogen oxygen or argon. Contact Air Products Technical Information Center at 800-654-4567.