Gases liquids or solids. The prefix bi- means two or double and carbonate refers to the polyatomic ion CO 3.

P 2 O 5.
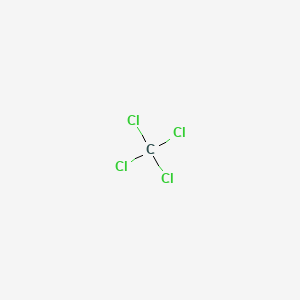
Carbon tetrachloride chemical formula. Carbon tetrachloride also known by many other names such as tetrachloromethane also recognised by the IUPAC carbon tet in the cleaning industry Halon-104 in firefighting and Refrigerant-10 in HVACR is an organic compound with the chemical formula CCl 4It is a colourless liquid with a sweet smell that can be detected at low levels. It is practically not flammable at lower temperatures. Carbon tetrachloride is a manufactured chemical that does not occur naturally.
It is a clear liquid with a sweet smell that can be detected at low levels. It is also called carbon chloride methane tetrachloride perchloromethane tetrachloroethane or benziformCarbon tetrachloride is most often found in the air as a. Carbon tetrachloride also known as tetrachloromethane is an organic compound with the chemical formula CCl4.
This compound is often classified as a polyhalogenated organic compound since it consists of a carbon atom which is attached to more than one halide functional group. Under standard conditions for temperature and pressure CCl4 exists as a colourless liquid which emanates a very. The chemical formula of sucrose is C12H22O11 meaning that each molecule of sucrose has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms.
The chemical formula for table salt otherwise known as sodium chloride is NaCl. Carbon forms compounds with the halogens with CX 4 as general formula where X is fluorine chlorine bromine or iodine. At ambient temperature carbon tetrafluoride is gas tetrachloride is liquid and the other two compounds are solids.
We also know mixed carbon tetrahalides. The most important of all may be the dichlorodifluoromethane CCl. The chemical formula for carbon tetrachloride is CCl 4.
The chemical name for baking soda is sodium bicarbonate. The symbol for sodium is Na. The prefix bi- means two or double and carbonate refers to the polyatomic ion CO 3.
The chemical formula therefore is NaCO 3 2. Try writing the formula for a compound named dinitrogen heptachloride. Di- means two or double so there are two nitrogen.
Carbo coal is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. Carbon makes up only about 0025 percent of Earths crust.
Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a radionuclide. Each chemical substance has a specific chemical composition so these chemical substances have their own chemical formula. Lets take a look at.
Covalent chemical bonds involve the sharing of a pair of valence electrons by two atoms in contrast to the transfer of electrons in ionic bonds. Such bonds lead to stable molecules if they share electrons in such a way as to create a noble gas configuration for each atom. Hydrogen gas forms the simplest covalent bond in the diatomic hydrogen molecule.
The halogens such as chlorine also exist. For example the molecule carbon tetrachloride is a non-polar covalent molecule CCl 4. Its melting point is -23C.
By contrast the ionic solid NaCl has a melting point of 800C. Crystalline solids made of ions High melting and boiling points Conduct electricity when melted Many soluble in water but not in nonpolar liquid. Gases liquids or solids.
Tin tetrachloride SnCl4 or Cl4Sn CID 24287 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Constitutional isomers have the same molecular formula but their physical and chemical properties may be very different.
For an example Click Here. Distinguishing Carbon Atoms When discussing structural formulas it is often useful to distinguish different groups of carbon atoms by their structural characteristics. A primary carbon 1º is one that is bonded to no more than one other carbon.
Chemical compounds are formed when elements are joined by chemical bonds. These bonds are so strong that the compound behaves like a single substance. Compounds have their own properties that are unique from the elements they are made of.
A compound is a type of molecule with more than one element. You can go here to learn more about molecules and compounds. CHAPTER 3 Chemical Equations Reaction Stoichiometry.
3 Objectives Understand how to write chemical equations Perform calculations based on chemical equations Calculate percent yields from chemical reactions Understand the concept of sequential reactions. 4 Equations Consider a simple equation. 2 3.
What happens to 2 if we multiply it by. A mixture of powdered magnesium with trichloroethylene or with carbon tetrachloride will flash or spark under heavy impact ASESB Pot. Stannic oxide heated with magnesium explodes Mellor 7401.
When carbon dioxide gas is passed over a mixture of powdered magnesium and sodium peroxide the mixture exploded Mellor 2490. Powdered magnesium plus potassium. 80 carbon tetrachloride _____ MULTIPLE CHOICE.
Choose the one alternative that best completes the statement or answers the question. 81 Which of the following pairs of elements would most likely form a ionic compound. A Ca and Ni B Cu and Ar C F and S D Zn and K E Na and Cl.
Each carbon atom can make 4 covalent bonds. These bonds can be to hydrogen atoms or to other carbon atoms. If all the covalent bonds between the carbon atoms in the hydrocarbon molecule are single bonds CC then the hydrocarbon molecule is said to be saturated.
The alkane homologous series C n H 2n2 provides many examples of saturated. N 2 F 6. P 2 S 3.
P 2 O 5. The catalyst formed by reaction of triethylaluminum with titanium tetrachloride has been widely studied but other metals eg. V Zr have also proven effective.
The following diagram presents one mechanism for this useful reaction. Others have been suggested with changes to accommodate the heterogeneity or homogeneity of the catalyst. Polymerization of propylene through action of the.
Because atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8. Carbon tetrachloride CCl 4 c methanol CH 3 OH d strontium fluoride SrF 2. Click here to check your answer to Practice Problem 1.
Use the following data to propose a way of distinguishing between ionic and covalent compounds. Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following.
Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3. 9 CO carbon monoxide.
For each of the following questions determine whether the compound is ionic or covalent and write the appropriate formula for it. 11 dinitrogen trioxide N2O3. 14 lithium acetate LiC2H3O2.
15 phosphorus trifluoride PF3. 16 vanadium V oxide V2O5. Silver perchlorate and carbon tetrachloride – when mixed in combination with hydrochloric acid forms a compound that detonates at 105F.
Formaldehyde – when mixed with hydrochloric acid forms a human carcinogen. Material reacts violently with bases and is corrosive with the generation of heat. Reacts with base metals forming combustible gas hydrogen.
Reacts violently with strong oxidants. PVDF Tables of Chemical Resistance Inorganic Media F 2 gas or liquid HBF 4 H 2SiF 6 N 2H 4H 2O HBr HCl HCl gas HF Hl H 2O 2 l 2 dry or moist FeCl 24H 2O FeCl 36H 2O FeNO 3 39H 2O FeSO 4 3 CH 3COO 2Pb3H 2O LiBr MgCO 3MgOH 23H 2O MgCl 26H 2O MgOH 2. Using our ever-expanding Chemical Guide you tell us your chemical we ask a few questions about how youd like your set-up to work and we design a customized system for you.
The Chemical Guide is the key to success. Once you tell us your chemical our system searches the 2000 chemicals in the Guide and tells you the correct materials of construction for your system from plastics metals.