
Give formulas for the following. Sulfur dioxide SO2_ 2.
Chemical Formula Nomenclature Practice.
Carbon disulfide formula. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities.
Occurrence manufacture properties. This page provided supplementary chemical data on carbon disulfide Material Safety Data Sheet. The handling of this chemical may incur notable safety precautions.
Carbon dioxide definition a colorless odorless incombustible gas CO2 present in the atmosphere and formed during respiration usually obtained from coal coke or natural gas by combustion from carbohydrates by fermentation by reaction of acid with limestone or other carbonates or naturally from springs. Used extensively in industry as dry ice or carbon dioxide snow in carbonated. Some of the most important compounds of carbon are carbon dioxide CO 2 carbon monoxide CO carbon disulfide CS 2 chloroform CHCl 3 carbon tetrachloride CCl 4 methane CH 4 ethylene C2H 4 acetylene C 2 H 2 benzene C 6 H 6 acetic acid CH 3 COOH and their derivatives.
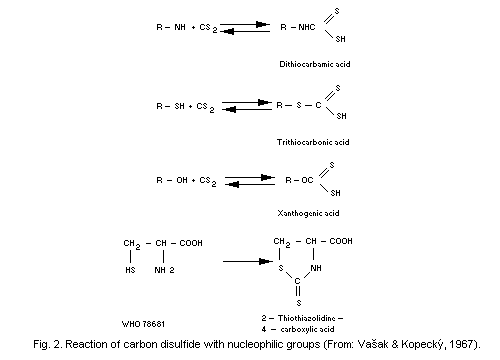
Los Alamos National Laboratory US. See more information at the Carbon compound. Molybdenum disulfide belongs to a class of materials called transition metal dichalcogenides TMDCs.
Materials in this class have the chemical formula MX 2 where M is a transition metal atom groups 4-12 in the periodic table and X is a chalcogen group 16. The chemical formula of molybdenum disulfide is MoS 2. The crystal structure of molybdenum disulfide MoS 2 takes the form of a.
From this formula it should be clear that nucleophiles may bond either at the carbonyl carbon as for any aldehyde ketone or carboxylic acid derivative or at the beta-carbon. These two modes of reaction are referred to as 12-addition and 14-addition respectively and will be displayed here when the Nucleophilic Addition button is clicked. What is the correct molecular formula for the compound antimony trichloride.
Sb 3 Cl. What is the correct molecular formula for the compound boron trifluoride. What is the correct molecular formula for the compound dinitrogen dichloride.
Suppose 120 g of carbon C reacts with 730 g of sulfur S to give 760 g of the compound carbon disulfide CS2. In the process all the carbon gets used up but some elemental sulfur is left o. CHAPTER 3 Chemical Equations Reaction Stoichiometry.
3 Objectives Understand how to write chemical equations Perform calculations based on chemical equations Calculate percent yields from chemical reactions Understand the concept of sequential reactions. 4 Equations Consider a simple equation. 2 3.
What happens to 2 if we multiply it by. Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. A good technical grade of carbon tetrachloride contains not more than the following amounts of impurities. 1 ppm acidity as HCl 1 ppm carbon disulfide if manufactured by carbon disulfide chlorination 20 ppm bromine 200 ppm water and 150 ppm chloroform.
The residue should not exceed 10 ppm on total evaporation. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I Mercury I sulfate N 2 O 3 M Dinitrogen trioxide CdS I Cadmium sulfide HC 2 H 3 O 2 A Acetic acid Sn 3 PO 4 2 I Tin II phosphate ZnCrO 4 I Zinc chromate KMnO 4 I Potassium permanganate C 2 H 6 M Dicarbon hexahydride MnO I Manganese II oxide CS 2 M Carbon disulfide PbCO 3 2 I Lead IV carbonate AuPO 4 I Gold II phosphate NH 3 M. CS2 carbon disulfide Br2O7 dibromine heptoxide CO carbon monoxide P2O3 diphosphorus trioxide Cl2O dichlorine monoxide SF6 sulfur hexafluoride Exercise.
Give formulas for the following. Iodine pentabromide IBr5 chlorine dibromide ClBr2 oxygen difluoride OF2 carbon tetrachloride CCl4 sulfur hexafluoride SF6 silicon dioxide SiO2 iodine heptafluoride IF7 nitrogen monoxide NO dinitrogen. Heat of Fusion Formula.
Customize your course in 30 seconds Which class are you in. Get started Get ready for all-new Live Classes. Now learn Live with Indias best teachers.
Join courses with the best schedule and enjoy fun and interactive classes. Sulfuric acid 98072 g. Chemical Formula Nomenclature Practice.
Complete these in lab and on your own time for practice. You should complete this by Sunday. Use the stock form for the transition metals.
Give the formula for the following. Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3.
El sulfuro de carbono o disulfuro de carbono CS 2 es un líquido volátil incoloro y muy fácilmente inflamableTienen un olor característico que empeora si está impuro debido a la hidrólisis parcial o total que libera ácido sulfhídrico H 2 S. Se mezcla completamente con la mayor parte de los disolventes orgánicos y disuelve el yodo azufre elemental fósforo blanco etc. 2 disulfide 3 Charge PO 3 3 phosphite PO 4 3 phosphate PO 2 3 hypophosphite AsO 3 3 arsenite AsO 4 3 arsenate 4 Charge P2O7 4 pyrophosphate Most commonly encountered ions in bold.
Polyatomic Ions - A group of atoms held together by covalent bonds found in ionic compounds. Know memorize recognize names formulas and charges. 2 Carbon disulfide 047589 028749 032260 55200 16158 55200 1256 23 CO 2 Carbon dioxide 046382 026160 029030 30419 21658 30419 0713 24 CO Carbon monoxide 029818 027655 029053 13292 6815 13292 25 CC 14 Carbon tetrachloride 056607 027663 029000 55635 25033 55635 1583 26 Cl 2 Chlorine 056600 027315 028830 41715 17212 41715 1398 27 CHC 13.
Empirical Formula Hill Notation. C 25 H 42 N 7 O 17 P 3 S xLi CAS No. 83762 free acid basis Compare Product No.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Properties of Organic Solvents.
The values in the table below except as noted have been extracted from online and hardbound compilations. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003.