
The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole. This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius.
How many grams of H_3PO_4.
Calcium oxide molar mass. Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compound. It is a white caustic alkaline crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium aluminium and iron predominate.
Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin.
This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius. CaOH 2 SO 4 CaSO 4 H 2 O. Soluble in water glycerol.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry.
All forms are white solids that are poorly soluble in water. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be atoms molecules or formula units of ionic compounds.
This relationship is frequently used in the laboratory. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole. The molar mass of sodium oxide is 6198 gmol.
2Na 1O 2299 gmol 16 gmol 6198 gmol. The molar mass of calcium sulfide is 7214 gmol. 1Ca 1S.
If 5 moles of NaCl weigh 2922 g what is the molar mass of table salt. As per the periodic table relative atomic mass of Sodium Na 2299 and Chlorine Cl 3545. Number of Moles frac MassMolar Mass Molar Mass frac MassNumber of Moles Molar Mass frac 29225 Molar Mass 5844 gmol.
74093 grams per mole. 2211 grams per cubic centimetre. White powder or colourless crystal.
CaOH 2 has a hexagonal crystal structure. It is not very soluble in water and its solubility reduces with an increase in temperature. For example its solubility at 0 o C is 189 gL and.
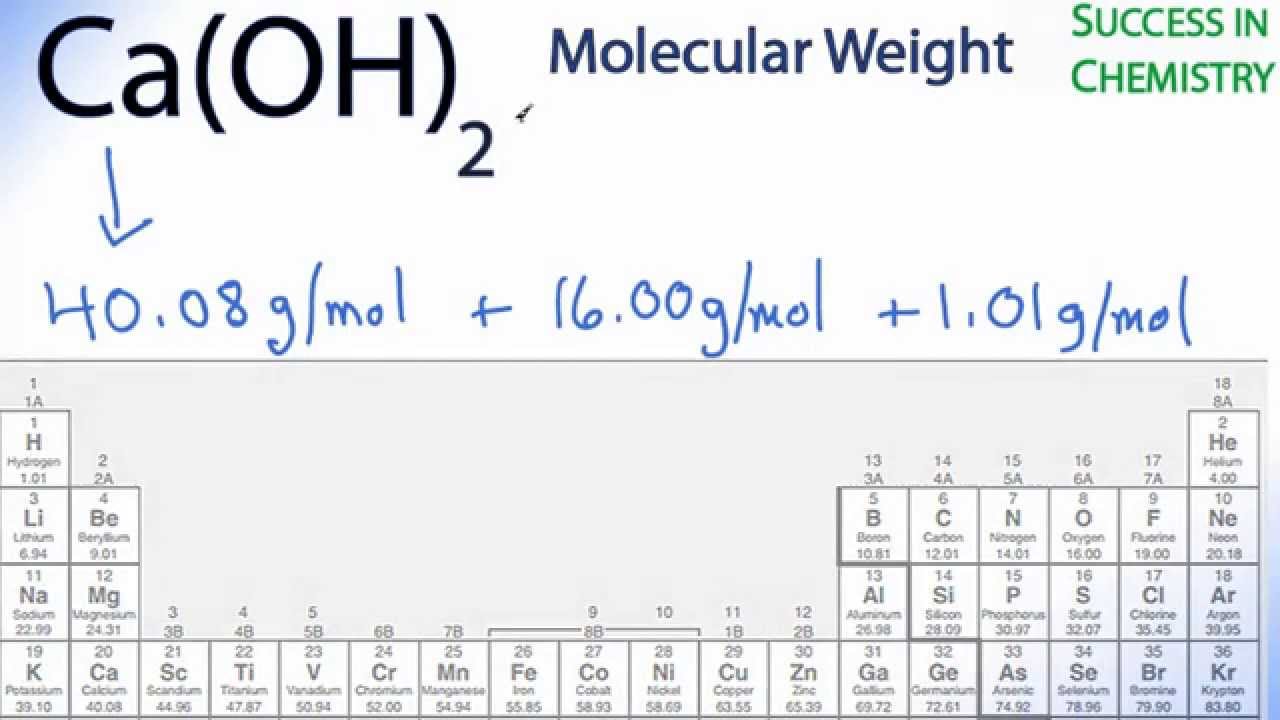
Aluminium oxide Al 2 O 3 Calcium nitrate CaNO 3 2 Potassium hydroxide KOH Copper II chloride CuCl 2 Sodium oxide Na 2 O Aluminium phosphate AlPO 4 Potassium sulphate K 2 SO 4 Calcium hydroxide CaOH 2 Magnesium carbonate MgCO 3 Sodium hydrogen carbonate NaHCO 3 Barium hydroxide BaOH 2 Chromium III oxide Cr 2 O 3. Balancing Chemical Equations Answer Key Balance the. Calcium Hydroxide CaOH 2.
Also commonly referred to as slaked lime or hydrated lime. Calcium hydroxide is formed as a result of hydrating lime calcium oxide CaO. Lime is by far the most economically favorable alkaline reagent to use for acid neutralization.
Lime is significantly cheaper than caustic NaOH but is much more difficult to handle. If the molar mass of the salt is 218 gmol what mass is required. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml.
How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. How many grams of H_3PO_4.