A water molecule is made up of two hydrogen atoms and one oxygen atom. Give the formula for the following.

E-mail to a friend.
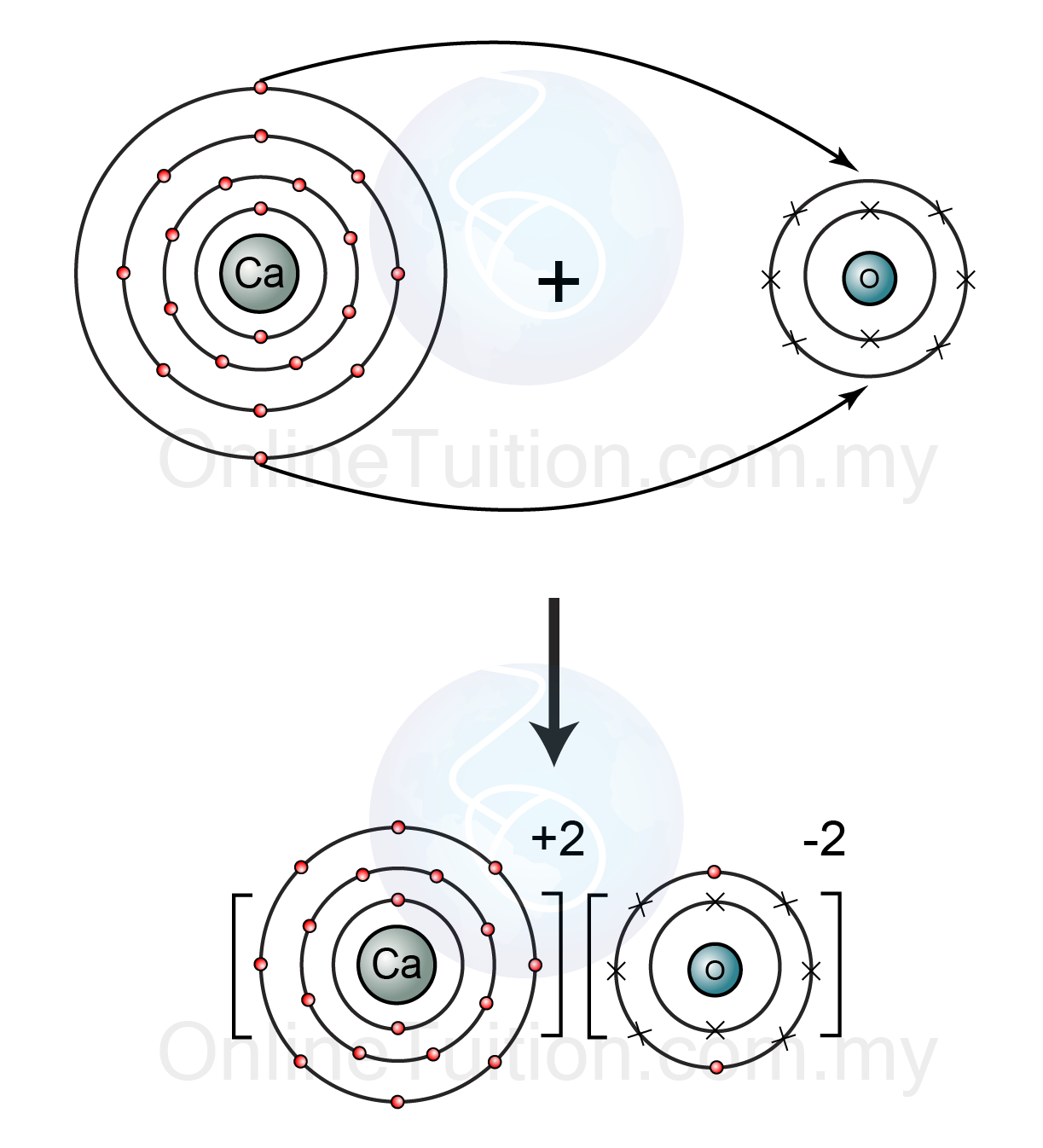
Calcium oxide formula. Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compoundIt is a white caustic alkaline crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium aluminium and iron predominate. Calcium oxide molecules contain one calcium cation which holds a charge of 2 and one oxygen anion which holds a charge of -2.
The structure of calcium oxide is illustrated below. Thus it can be understood that calcium oxide is an ionic compound featuring an ionic bond between calcium and oxygen. Calcium oxide CaO used in the pulp manufacturing.
Chemical is part of scrap metaliron kish used in the manufacture of metal in an electric arc furnace. Corrosion inhibitors and anti-scaling agents. Engineered fill for construction.
Graveling and road bed material. At 1200K calcium carbonate decomposes to give carbon dioxide and calcium oxide. CaCO 3 CaO CO 2.
On reacting with dilute acids calcium carbonate gives carbon dioxide. CaCO 3 2HC l CaCl 2 H 2 O CO 2. Application of Calcium Carbonate.
Calcium carbonate is largely employed in the pulp and paper industry. It can be used as a filter and pigment making possible the production of a. Calcium is a chemical element with the symbol Ca and atomic number 20.
As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earths crust and the third most abundant metal after iron and.
To write the chemical formula for Manganese IV Oxide you must make sure to balance the charges. The roman numeral IV in front of manganese tells us that it is oxidizing at 4Looking at the table oxygen is a group 16 element which means it oxidizes at a 2- state To neutralize the oxidation number of manganese two atoms of oxygen should be added to achieve the final compound. Ionic Compound Naming and Formula Writing List 1.
Copy this to my account. E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
Ionic Compounds Naming and Formula Writing. Copy this to my account. E-mail to a friend.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Vitamin C as Calcium Ascorbate Vitamin D as D3 Cholecalciferol Calcium Elemental from Microcrystalline Hydroxyapatite Magnesium as Magnesium Oxide Zinc as Zinc L-Methionine Sulfate Copper as Copper Gluconate Manganese as Manganese Citrate Potassium as Potassium Citrate Vitamin K2 as Natural MK-7 Menaquinone-7 Boron as Boron Citrate cellulose capsule bovine. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
Metallic calcium was first isolated by Sir Humphry Davy in 1808 through the electrolysis of a mixture of lime CaO and mercuric oxide HgO. Today metallic calcium is obtained by displacing calcium atoms in lime with atoms of aluminum in hot low-pressure containers. About 42 of the earths crust is.
Its formulation employs calcium citrate to deliver the promised calcium as well as magnesium oxide and zinc oxide for the other three minerals included in the tablets. Each tablet provides 250 mg of calcium 40 mg of magnesium and 5 mg of zinc. Not only that it can hold its own in the analytical lab too.
The analytically determined calcium content is exactly 250 mg per tablet though its. The chemical formula of ionic compounds can be quickly calculated using the chemical formula. Sodium chloride NaCl and magnesium oxide MgO.
The transfer of electrons between metals and non-metals produces charged particles called ions. Metals lose electrons to produce positve ions called cations. Na Mg 2 Non-metals gain electrons to produce negative ions called anions.
Cl- O 2. Deuterium oxide D 2 O called heavy water is important in chemical research and is also used as a neutron moderator in some nuclear reactors. A water molecule is made up of two hydrogen atoms and one oxygen atom.
A single oxygen atom contains six electrons in its outer shell which can hold a total of eight electrons. When two hydrogen atoms are bound to an oxygen atom the. Our Enfamil Baby Formula Powder features a range of different size options.
You can conveniently purchase this Baby Formula in either powder or concentrate form. The powder product is available in 211 oz 294 oz and 125 oz sized cans and the concentrate form in 13 fl oz. You can also purchase Enfamil Baby Formula Powder in a bulk order with our convenient case of 4 case of 6 and.
Bone Metabolism Formula with Minerals. Family Owned Since 1968. GMP Quality Assured.
Calcium citrate is a readily digested and absorbed form of calcium a mineral that is necessary for the maintenance of bone health. Vitamin D magnesium zinc copper and manganese have been included for their. If a question is asking about the high melting point of magnesium oxide a student will not get much credit for just saying it has strong bonds or strong ionic bonds.
A good answer might be. MgO has a giant lattice structure with strong electrostatic forces of attraction between oppositely charged ions. These require a lot of energy to break.
Similarly if talking about hydrogen bonding in. Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following.
Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3.