In its solid form ice water is less dense than when it is liquid another unusual. SnS tin II sulfide 8.

CuO copper II oxide 15.
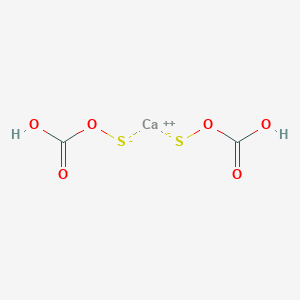
Calcium hydrogen sulfide formula. Hydrogen sulfide is a chemical compound with the formula H 2 S. It is a colorless chalcogen hydride gas with the characteristic foul odor of rotten eggs. It is poisonous corrosive and flammable.
Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen such as in swamps and sewers. This process is commonly known as anaerobic digestion which. Ionic Compound Naming and Formula Writing List 1.
Copy this to my account. E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Sulfide British English also sulphide is an inorganic anion of sulfur with the chemical formula S 2 or a compound containing one or more S 2 ions. Solutions of sulfide salts are corrosive.
Sulfide also refers to chemical compounds large families of inorganic and organic compounds eg. Lead sulfide and dimethyl sulfide. Hydrogen sulfide H 2 S and bisulfide SH are the conjugate.
Match each formula with the correct name. MgS 1 magnesium sulfite MgSO 3 2 magnesium sulfate MgSO 4 3 magnesium sulfide B. CaClO 3 2 1 calcium chlorate CaCl 2 2 calcium chlorite CaClO 2 2 3 calcium chloride Learning Check.
Although its formula H 2 O seems simple water exhibits very complex chemical and physical properties. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds such as hydrogen sulfide and ammonia. In its solid form ice water is less dense than when it is liquid another unusual.
Hydrogen is a colorless odorless gas. It is easily ignited. Once ignited it burns with a pale blue almost invisible flame.
The vapors are lighter than air. It is flammable over a wide range of vaporair concentrations. This formula points out that in one molecule of the compound 18 carbon atoms 21 hydrogen atoms one nitrogen atom and three oxygen atoms are existent.
There are ten calcium atoms. The amounts of phosphorous hydrogen and oxygen are affected by the subscripts outside the parentheses. There are six phosphorous atoms and two hydrogen atoms.
H hydrogen. K potassium. Sr 2 strontium.
Some metals form positive ions in more than one oxidation state. One of the earliest methods of distinguishing between these ions used the suffixes -ous and -ic added to the Latin name of the element to represent the lower and higher oxidation states respectively. Fe 2 ferrous.
Fe 3 ferric. Sn 2 stannous. Sn 4 stannic.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Cadmium sulfide CdS 810 28. Calcium carbonate CaCO 3 2810 9.
Calcium chromate CaCrO 4 7110 4. Calcium fluoride CaF 2 5310 9. Calcium hydrogen phosphate CaHPO 4 110 7.
Calcium hydroxide CaOH 2 5510 6. Calcium oxalate CaC 2 O 4 2710 9. Calcium phosphate Ca 3 PO 4 2 2010 29.
Calcium sulfate CaSO 4. NH42S ammonium sulfide K3PO4 potassium phosphate ZnNO32 zinc nitrate Fe2SO43 ironIII sulfate or ferric sulfate CuCO3 copperII carbonate or cupric carbonate Note. In a formula parentheses are used around a polyatomic ion only when there are 2 or more of that polyatomic ion in a formula unit ie when the subscript is not 1.
HSO 4-Hydrogen carbonate or Bicarbonate. Cr 2 O 7 2-Thiosulfate. S 2 O 3 2-Carbonate.
C 2 O 4 2-Peroxide. O 2 2–3 ions. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. If you know the acid formula you will always get the correct anion formula and its charge since the charge is equal to the number of ionizable hydrogen atoms in the acid and is always negative.
For example for sulfuric acid H 2 SO 4 the anion is sulfate SO 4 2- with a -2 charge. Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following.
Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3. HPO 4 2-Dihydrogen phosphate.
H 2 PO 4-Sulfate. S 2 O 3 2-Sulfite. CrO 4 2-Hydrogen carbonate or.
SnS tin II sulfide 8. Ca3N2 calcium nitride 9. Hg2I2 mercury I iodide 10.
PbO lead II oxide 11. PbBr2 lead II bromide 12. HgS mercury II sulfide 13.
CaF2 calcium fluoride 14. CuO copper II oxide 15. Cu3P2 copper II phosphide 16.
KCl potassium chloride 17. SnS2 tin IV sulfide 18. Cd3N2 cadmium nitride 19.
ZnF2 zinc fluoride 20. There are three elements - carbon hydrogen and oxygen - so find their atomic masses in the periodic table. 120107 grams per mol for carbon 100794 grams per.
Empirical Formula Hill Notation. C 25 H 42 N 7 O 17 P 3 S xLi CAS No. 83762 free acid basis Compare Product No.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Write the formula for the conjugate base of each of the following acids.
Sulfurous acid H 2 SO 3 b. Chloric acid HClO 3 c. Hydrogen sulfide H 2 S.
Dimethyloxonium CH 3 2 OH e. Hydrogen sulfate HSO 4-Solution. Write the formula for the conjugate acid of each of the following bases.
Dimethylamide CH 3 2 N-b. Sulfide S 2-c. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.