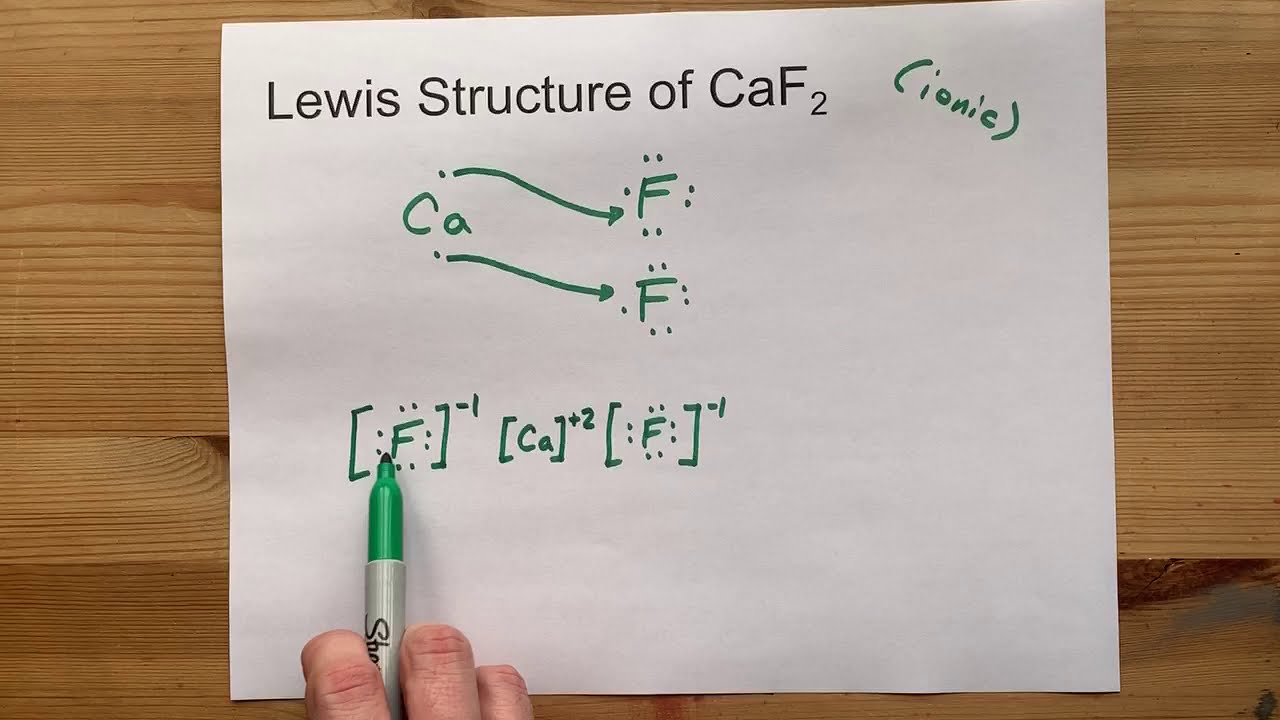
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF 2. Lead IV oxide PbO2.

Fluoride salts typically have distinctive bitter tastes and are odorless.
Calcium fluoride formula. Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF 2. It is a white insoluble solid. It occurs as the mineral fluorite also called fluorspar which is often deeply coloured owing to impurities.
The compound crystallizes in a cubic motif called the fluorite structure. Unit cell of CaF 2 known as fluorite structure from. Gas chromatography was used to measure the maternal and fetal plasma inorganic fluoride values at term in 91 women.
They were assigned to one of four groups. Group A were untreated controls. Group B received a single daily dose of 15 mg of fluoride as calcium fluoride during the final trimester of pregnancy.
Group C was given a single dose of 15 mg of fluoride as sodium fluoride and. Calcium fluoride has a low absorption coefficient and high damage threshold making these windows a good choice for use with free-space lasers. Optical calcium fluoride offers low dispersion with an Abbe Number of 95 and low fluorescence as well as excellent water chemical and heat resistance.
In dry environments CaF 2 can be used up to 1000 C but in the presence of moisture. Fluoride ˈ f l ʊər aɪ d ˈ f l ɔːr- is an inorganic monatomic anion of fluorine with the chemical formula F also written F whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes and are odorless.
Its salts and minerals are important chemical reagents and industrial chemicals mainly used in the production of hydrogen. Ionic Compound Naming and Formula Writing List 1. Copy this to my account.
E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Adding fluoride says the ADA is like fortifying milk with vitamin D orange juice with calcium or cereals with B vitamins and folic acid.
Studies continue to show that adding fluoride to water. Ionic Compounds Naming and Formula Writing. Copy this to my account.
E-mail to a friend. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Extended Release Formula continuously releasing fluoride up to 24 hours.
Contains 22600 ppm fluoride and tri-calcium phosphate TCP Advanced protection virtually invisible and durable long-lasting coating. Easy to apply no drying needed. Fast horizontal sweep application.
Can be applied to tooth surfaces where plaque is present. Unique applicator for back-of-glove dispensing. The metal has a silvery color is rather hard and is prepared by electrolysis of fused chloride and calcium fluoride to lower the melting point.
Chemically it is one of the alkaline earth elements. It readily forms a white coating of nitride in air reacts with water burns with a yellow-red flame. Los Alamos National Laboratory US.
Fluoride protects teeth from the bacteria in plaque. It also promotes new bone formation. This is different than most medicines used for weak bones osteoporosis which fight osteoporosis by.
The formula of HA shows the sites for atomic substitution. This HA is calcium deficient and carbonated. X calcium substitution.
Calcium phosphate and fluoride ions play an important role in the battle between demineralization and remineralization processes and accordingly modify the susceptibility of tooth to caries progression. 62 During demineralization calcium release precedes. Important isotopes of calcium include 48 Ca 46 Ca 44 Ca 43 Ca 42 Ca and 40 Ca.
Traced in large amounts as gypsum calcium sulfate limestone calcium carbonate apatite calcium chloro- or fluoro-phosphate and fluorite calcium fluoride. Dissolved calcium bicarbonate is found in hard water. Certain Facts About Calcium.
Formula-fed babies require additional intakes in the vicinity of 350 mgday as calcium is less bioavailable in formula. The AI for infants 7-12 months was set by adding an estimate for calcium from breast milk at this age to an estimate of intake from supplementary foods. A breast milk volume of 060 Lday was assumed at older ages Dewey et.
Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid CAS No. DOT ID Guide. 1052 125anhydrous 1790 157solution Formula.
1 ppm 082 mgm 3. NIOSH REL TWA 3 ppm 25 mgm 3 C 6 ppm 5 mgm 3 15-minute OSHA PEL TWA 3 ppm See Appendix G. H 0 1 2.
Calcium Fluoride CaH2 Calcium Hydride CaI2 Calcium Diiodide CaO Calcium Oxide CaS Calcium Sulfide CaSO4 Calcium Sulfate CBr4 Carbon Tetrabromide CCl2F2 Dichlorodifluoromethane CCl4 Carbon Tetrachloride CdNO32 Cadmium Nitrate CdS Cadmium Sulfide CF4 Carbon Tetrafluoride CH2Cl2 Dichloromethane. Water fluoridation is the water adjustment process that rises or reduces the natural fluoride concentration in drinking water to an optimum level thats effective for reducing tooth decay and promoting good dental health. Its done to aid in both dental and skeletal health or more specifically to reduce and prevent the prevalence of tooth damage and decay across.
Al 2 O 3. Cr 2 H 8 N 2 O 7. C 2 H 3 O 2 NH 4.
NH 4 HCO 3. Solubility Product Constants near 25 C. Ionic Compound Formula K sp.
Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Calcium is a mineral involved. Studies on water fortification include examples of fortification of public water supplies with fluoride to prevent dental cavities that have been implemented for more than 50 years in more than 25 countries.
However most evidence of its effects comes from observational studies 108109110111. There are some studies in Asia regarding iodized water. Lead II fluoride PbF2 76.
Calcium nitrate CaNO32 66. Chromium III sulfate Cr2SO43 77. Zinc hydroxide ZnOH2 67.
Ammonium chlorate NH4ClO3 78. Sodium carbonate Na2CO3 68. Mercury II chromate HgCrO4 79.
Lead IV oxide PbO2. A low fluoride formula reduces the chances of fluorosis and associated white spots and brown stains. BioMin F for Kids also contains bio-available calcium and phosphate which are essential in driving the remineralising process and reversing early decay.
BioMin F vs BioMin F for Kids. Mint flavouring has been replaced with a strawberry flavour designed for the pallets of children 3-6. Fluoride is negatively charged and combines with positive ions eg calcium or sodium to form stable compounds eg calcium fluoride or sodium fluoride.
Such fluorides are released into the environment naturally in both water and air. Fluoride compounds also are produced by some industrial processes that use the mineral apatite a mixture of calcium phosphate compounds. For Water Treatment and Water Distribution.
Measurement Conversion Measurement Conversion Measurement Conversion Measurement Conversion 1 ft. 1 MGD 155 cfs 1 grain gal 171 mgL 1 min 60 sec 1 yd. Calcium a mineral you may be familiar with is a macro mineral that plays a vital role in strong bones and helps muscles relax and contract.
Calcium is well-known for being in dairy products but it can also be found in many other foods like salmon or greens like broccoli. If youre not a big fan of dairy products or fish you can also find calcium in other sources such as fortified tofu or. CaF2 calcium fluoride 14.
CuO copper II oxide 15. Cu3P2 copper II phosphide 16. KCl potassium chloride 17.
SnS2 tin IV sulfide 18. Cd3N2 cadmium nitride 19. ZnF2 zinc fluoride 20.
Cs 2O cesium oxide 21. LiI lithium iodide 22. Hg2F2 mercury I fluoride 23.
CuCl copper I chloride 24. Ca3P2 calcium phosphide 25. Na2N sodium nitride 26.
SnO tin II oxide 27. Fe2O3 iron III oxide 28.