
EDTA is ethylene diamine tetraacetic acid or H 4C 10H 12N 2O 4. Indeed the versatility of water as a solvent is essential to living organisms.
Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states.
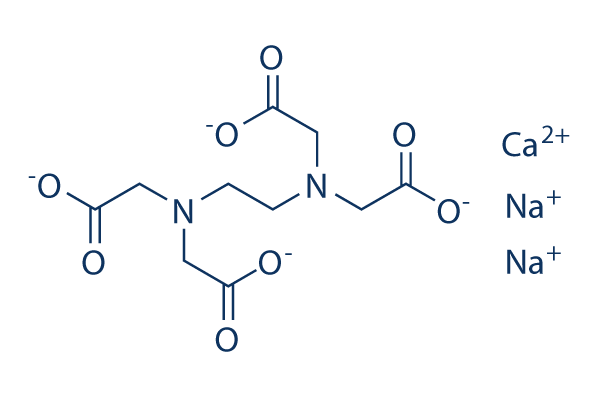
Calcium disodium edta. Calcium disodium EDTA is an odorless crystalline powder with a slightly salty flavor Its a popular food additive used as a preservative and flavoring agent. Calcium disodium EDTA works as. Ethylenediaminetetraacetic acid EDTA is an aminopolycarboxylic acid with the formula CH 2 NCH 2 CO 2 H 2 2This white water-soluble solid is widely used to bind to iron and calcium ions.
It binds these ions as a hexadentate six-toothed chelating agentEDTA is produced as several salts notably disodium EDTA sodium calcium edetate and tetrasodium EDTA. EDTA is a chemical that binds and holds on to chelates minerals and metals such as chromium iron lead mercury copper aluminum nickel zinc calcium cobalt manganese and magnesium. Cosmetic Ingredient Expert Review Panel.
Final Final Report on the Safety Assessment of EDTA Calcium Disodium EDTA Diammonium EDTA Dipotassium EDTA Disodium EDTA TEA-EDTA Tetrasodium EDTA Tripotassium EDTA Trisodium EDTA HEDTA and Trisodium HEDTA. International Journal of Toxicology 21 S2. 95-142 2002 Hazardous Substances Data Bank HSDB LD50 Rabbit iv 47 mgkg.
Cosmetic Ingredient Expert Review Panel. Final Final Report on the Safety Assessment of EDTA Calcium Disodium EDTA Diammonium EDTA Dipotassium EDTA Disodium EDTA TEA-EDTA Tetrasodium EDTA Tripotassium EDTA Trisodium EDTA HEDTA and Trisodium HEDTA. International Journal of Toxicology 21 S2.
95-142 2002 Hazardous Substances Data Bank HSDB LD50 Rabbit iv 47 mgkg. EDTA is usually given for 5 days in a row followed by 1 day off the medicine. This schedule is then repeated for as long as needed until blood calcium levels are lowered to a safe level.
After you receive EDTA you will need to remain lying down for a short time. Reversing high blood levels of calcium can cause a drop in your blood pressure. Ethylenediaminetetraacetic acid EDTA edetate calcium disodium calcium disodium versenate is a chelation agent used for heavy metal toxicity.
EDTA which was first synthesized in the mid-1930s has non-pharmacologic and pharmacologic purposes. As a pharmacologic agent EDTA is used as calcium disodium edetate which prevents it from binding calcium in the body. Calcium ions changing colour from blue to pinkred in the process but the dyemetal ion complex is less stable than the EDTAmetal ion complex.
As a result when the calcium ionPR complex is titrated with EDTA the Ca2 ions react to form a stronger complex with the EDTA. For the titration the indicator is added to the sample solution. Ethylenediamine tetraacetic acid EDTA.
FDC colors All G. GMP disodium guanylate gold leaf. Hexa- hecta and hepta-esters of sucrose.
High fructose corn syrup. Disodium EDTA is quite common and largely used in food skincare and cosmetic industries. Its a staple in most products.
Particularly for food and skincare products adding disodium EDTA into the formula can be quite controversial even with FDA approvals for the ingredient as a food additive theres a set of limitations of how much of the powder can be added into the products - a good. Calcium ions can be analyzed by titration with EDTA using an appropriate indicator. EDTA is ethylene diamine tetraacetic acid or H 4C 10H 12N 2O 4.
EDTA itself is not very water soluble so the disodium salt is used Na 2H 2C 10H 12N 2O 4. For the purpose of simplicity Y will stand for C 10H 12N 2O 4. The EDTA we use is thus Na 2H 2Y.
The part that reacts with calcium ions is H 2Y 2-according. Solid EDTA is available in a form the disodium salt which is sufficiently pure to be used as a primary standard. However in this experiment in which the highest possible level of accuracy is desired we will standardize the EDTA solution with primary standard solid calcium carbonate.
This will also provide useful practice in detecting the end point before analysis of your unknown solutions. Buy an official bottle of Olive Garden Salad Dressing at a nearby location. Dont settle for imitation recipes or copycats.
Tetrasodium EDTA is the salt resulting from the neutralization of ethylenediaminetetraacetic acid with four equivalents of sodium hydroxide or an equivalent sodium base. It is a white solid that is highly soluble in water. Commercial samples are often hydrated eg.
The properties of solutions produced from the anhydrous and hydrated forms are the same provided they are. Par exemple dans le DMNP-EDTA utilisé en biologie pour étudier des cellules vivantes lEDTA chélate le calcium ou dautres ions. 139-33-3 6381-92-6 EDTA-TEA TriÉthanolAmine-EDTA-TEA.
60544-70-9 EDTA-Zinc Zn 92143-36-7. Calcium disodium EDTA is a common food additive and an ingredient in cosmetic and industrial products. This article reviews calcium disodium EDTA.
Calcium disodium EDTA label claims to protect flavor however its purpose more accurately stated is to prevent benzene formation by chelating the metal ions present in water that can act as catalysts in the reaction between sodium benzoate and erythorbic acid Brominated vegetable oil. Diminishing bone mass also triggers a rise in calcium levels in the blood which increases the risk of kidney stones. Researchers suspect the root cause of bone loss in space is weightlessness.
The pull of gravity 350 km above our planets surface – where the space station and the shuttle orbit – is 90 percent as strong as it is on the ground. That hardly sounds weightless. EDTA is a molecule called a chelating agent.
A chelating agent is a claw-like substance that can grab and stick to other molecules. Some types of EDTA stick to calciumOther types stick to metals. Its especially useful for sequestering calcium Ca 2 and iron Fe 3 metal ions.
This is the lab recipe for 05 M EDTA solution at pH 80. 1861 grams EDTA disodium ethylenediaminetetraacetate2H 2 O 800 milliliters distilled water. Sodium hydroxide NaOH solution or solid to adjust pH Procedure.
Stir 1861 g disodium ethylenediaminetetraacetate2H 2 O into. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states. It is one of the most plentiful and essential of compoundsA tasteless and odourless liquid at room temperature it has the important ability to dissolve many other substances.
Indeed the versatility of water as a solvent is essential to living organisms. Calcium disodium EDTA chelation removes heavy metals and minerals from the blood such as lead iron copper and calcium and is approved by the FDA for use in treating lead poisoning and toxicity from other heavy metals. Rather than testing calcium disodium EDTA TACT used another salt disodium EDTA under an FDA license as an Investigational New Drug IND.
Although disodium EDTA it is not. Detection and quantification of coronary artery calcium CAC scores with electron beam tomography has been shown to correlate with obstructive and nonobstructive coronary artery. Calcification in coronary artery disease can be reversed by EDTA-tetracycline long-term chemotherapy Pathophysiology.
Aqua Water Eau Glycerin CaprylicCapric Triglyceride Niacinamide Cetearyl Alcohol Potassium Phosphate Ceramide NP Ceramide AP Ceramide EOP Carbomer Dimethicone Ceteareth-20 Behentrimonium Methosulfate Sodium Lauroyl Lactylate Sodium Hyaluronate Cholesterol Phenoxyethanol Disodium EDTA Dipotassium Phosphate Caprylyl Glycol Phytosphingosine Xanthan.