Simple Binary Ionic Compounds Please complete the following table. Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N.

Calcium carbonate calcite CaCO 3 336 109 Calcium fluoride CaF 2 345 1011 Calcium hydroxide CaOH 2 502 106 Compound Formula K sp Calcium iodate CaIO 3 2 647 106 Calcium iodate hexahydrate CaIO 3 2 6H 2 O 710 107 Calcium molybdate CaMoO 4 146 108 Calcium oxalate monohydrate CaC 2 O 4 H 2 O 232 109 Calcium phosphate Ca 3 PO.
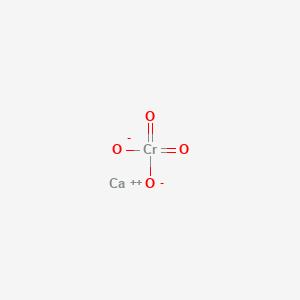
Calcium chromate formula. CrO 2 4 and Cr 2 O 2 7 Molar mass. 115994 g mol 1 and 215988 g mol 1 Conjugate acid. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
Chromate salts contain the chromate anion CrO 2 4. Dichromate salts contain the dichromate anion Cr 2 O 2 7. Ionic Compounds Naming and Formula Writing.
Copy this to my account. E-mail to a friend. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
Polyatomic ions are ions which consist of more than one atom. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. Calcium is a chemical element with the symbol Ca and atomic number 20.
As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earths crust and the third most abundant metal after iron and.
Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. 8 water molecules octahydrate. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot.
CaCO3 calcium carbonate 11. BaSO4 barium sulfate 2. FeO iron II oxide 12.
Write the chemical formula for each of the following compounds. Sodium nitrite NaNO2 31. Potassium carbonate K2CO3 22.
Iron III oxide Fe2O3 32. Silver sulfide Ag2S 23. Aluminum hydroxide AlOH3 33.
Nickel II carbonate NiCO3 24. Ammonium hydroxide NH4OH 34. Ionic Compound Formula K sp.
Aluminum hydroxide AlOH 3 1810 5. Aluminum phosphate AlPO 4 6310 19. Barium carbonate BaCO 3 5110 9.
Barium chromate BaCrO 4 1210 10. Barium fluoride BaF 2 1010 6. Barium hydroxide BaOH 2 510 3.
Barium sulfate BaSO 4 1110 10. Barium sulfite BaSO 3 810 7. Barium thiosulfate BaS 2 O 3 1610 6.
Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver Sulfate AgBr Silver Bromide AgCh3COO Silver Acetate AgCl Silver Chloride AgF SilverI Fluoride AgNO3 Silver Nitrate AlNO33 Aluminium Nitrate AlOH3. The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Empirical formulae are usually obtained based on the analysis of experimental data.
The empirical formula for glucose is CH 2 O. Empirical Formulae can be derived from the molecular formulae. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating.
Potassium chromate is highly corrosive and is a strong oxidizing agent. This substance is used in the manufacture of dyes and in textile dyeing processes. Potassium chromate primarily affects the nose throat and lungs causing.
Simple Binary Ionic Compounds Please complete the following table. Formula of Ionic Compound s 2. Name of Ionic Compound.
Chemical formula plays an important role in understanding different concepts of chemistry. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
Chemical formulas can be. Sodium chromate is produced commercially by roasting chromite ore with sodium carbonate or with sodium carbonate and calcium oxide and leaching to dissolve the sodium chromate. After treatment to remove hydrated alumina the sodium chromate solution is either marketed directly or evaporated to produce hydrated or anhydrous crystals.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Formula writing rules to write the correct chemical formulas for each compound. Compound Name Type of Compound.
4 ammonium chromate 25 CuO copper II oxide 26 Pt 3PO 3 4 platinum IV phosphite 27 AlClO 4 3 aluminum perchlorate 28 NH 3 ammonia 29 CaC 2H 3O 2 2 calcium acetate 30 N 2O dinitrogen monoxide 31 VSO 4 2 vanadium IV sulfate 32 Ag 2CO. Calcium carbonate calcite CaCO 3 336 109 Calcium fluoride CaF 2 345 1011 Calcium hydroxide CaOH 2 502 106 Compound Formula K sp Calcium iodate CaIO 3 2 647 106 Calcium iodate hexahydrate CaIO 3 2 6H 2 O 710 107 Calcium molybdate CaMoO 4 146 108 Calcium oxalate monohydrate CaC 2 O 4 H 2 O 232 109 Calcium phosphate Ca 3 PO. K sp at 25 o C.
BaOH 2 x 8H 2 O. BaIO 3 2 x H 2 O. Formula and the correct name of the chemical compound.
The reward for building a correct chemical compound is receiving a number of points based on the number value of the cards played ie. Sodium chloride would score 2 points 1 point for the cation and one for the anion. Similarly calcium chloride would score 4 points two.
44 calcium bromide CaBr 2 45 gallium III chloride GaCl 3 46 sodium hydride NaH 47 beryllium hydroxide BeOH 2 48 zinc carbonate ZnCO 3 49 manganese VII arsenide Mn 3 As 7 50 copper II chlorate CuClO 3 2 51 cobalt III chromate Co 2 CrO 4 3 52 ammonium oxide NH 4 2 O 33 potassium hydroxide KOH. Calcium chloride is made of calcium ions with 2 charges Ca and chloride ions with 1 charge only Cl. You need 2 chloride anions to balance each calcium cation.
Therefore the formula of calcium chloride is CaCl2. Similarly in sodium carbonate you have sodium cations Na and carbonate anions CO 3 so. Students enrolled in Dr.
Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below. Chemical or scientific names are used to give an accurate description of a substances composition. Even so you rarely ask someone to pass the sodium chloride at the dinner table.
Its important to remember that common names are inaccurate and vary from one place and time to another. CaSO4 calcium sulfate 5. K2Cr2O7 potassium dichromate 6.
KHCO3 potassium bicarbonate 7. Cu2CO3 copper I carbonate 8. FeNO22 iron II nitrite 9.
AgNO3 silver nitrate 10. BaClO42 barium perchlorate 11. PbCO32 lead II carbonate 12.
CdOH2 cadmium hydroxide 13. SnSO4 tin II sulfate 14. ZnHCO32 zinc bicarbonate 15.
Hg2NO22 mercury I nitrite 16. 24 calcium bromide CaBr2 25 gallium chloride GaCl3 26 sodium hydride NaH 27 beryllium hydroxide BeOH2 28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium.