Caesium Peroxide Cs 2 O 2. Study the full list of natural healthy ingredients BLUE makes their pet food with from ALFALFA to ZINC.
Some common reactions are.
Calcium chloride reactions. It affects flavor and chemical reactions during the brewing process and it can also affect yeast function during fermentation. Calcium chloride can be injected as intravenous therapy for the treatment of hypocalcemia low serum calcium. It can be used for insect bites or stings such as Black Widow spider bites sensitivity reactions particularly when characterized by urticaria.
The process involves two parallel reactions. Calcium chloride CaCl 2 is a salt that is marketed in pellet form for removing dampness from rooms. Calcium chloride is also used for maintaining unpaved roads and for fortifying roadbases for new construction.
In addition calcium chloride is widely used as a de-icer since it is effective in lowering the melting point when applied to ice. Here we will understand how AlCl3 reacts with other compounds. If we take anhydrous aluminium chloride it is a powerful Lewis acid.
What this means is that it is capable of forming Lewis acid-base adducts even with bases that are weak in nature. For example mesitylene and benzophenone. Some common reactions are.
AlCl 3 can form tetrachloroaluminate AlCl 4. The main chemical in single use hot packs is either calcium chloride or magnesium sulfate. The other ingredient is distilled water.
Calcium chloride is a common salt that is often used to melt ice from roads and sidewalks. Calcium chloride isnt a food-grade salt but it isnt toxic. High concentrations of calcium chloride can harm plants and trees near roadways.
Calcium sulfate or calcium sulphate. Hydration and dehydration reactions. Or pink hydrated due to impregnation with cobaltII chloride which functions as a moisture indicator.
Up to the 1970s commercial quantities of sulfuric acid were produced in Whitehaven Cumbria UK from anhydrous calcium sulfate. Upon being mixed with shale or marl and roasted the sulfate liberates sulfur. Calcium chloride is shipped in three basic forms.
1 flake pebble and powdered form containing 77 to 80 calcium chloride 2 solid crystallized form containing 73 to 75 calcium chloride and 3 water solutions or liquors in any percentage with most common being 71 45 and 32. Potassium and chlorine gas — chloride. Lime carbon dioxide — calcium carbonate used to strengthen masonry water carbon dioxide light — glucose and oxygen photosynthesis sodium chloride — sodium chloride table salt Combustion reactions are often included in lists of synthetic reactions.
Combustion occurs when a chemical combines with oxygen. The product is usually fire. The complete glossary of wholesome ingredients and their BENEFITS Blue Buffalo uses in its dog and cat food.
Study the full list of natural healthy ingredients BLUE makes their pet food with from ALFALFA to ZINC. Includes definitions of what each ingredient is andor why its beneficial. 10 mLminute 136 meqminute.
Adverse Reactions Patients may complain of tingling sensations a sense of oppression or heat waves and a calcium or chalky taste following the intravenous administration of calcium gluconate. Rapid intravenous injection of calcium salts may cause vasodilation decreased blood pressure bardycardia. Click Here for MCQ video of Chemical Reactions and Equations.
Q1- Reaction of magnesium with air is A Exothermic reaction B Endothermic reaction C Reversible reaction D Substitution reaction. Q2- What chemicals are used in fireworks. A Copper chloride B Calcium chloride C Barium chloride D All of above.
CBSE Class 10 English MCQs. Q3- When a magnesium ribbon is burnt. Calcium Carbide CaC 2.
Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride. Zirconium Oxide with Calcium Impurity. Calcium Carbonate CaCO 3 Polymorphs.
Li 2 NH antifluorite LiNH 2 defect antifluorite Group 1 Elements. Caesium Peroxide Cs 2 O 2. Dipotassium Pentasulfide K 2 S 5.
The chemical reaction between baking soda and calcium chloride. Using the language of breaking and making bonds explain the net energy change for the chemical reaction between baking soda and calcium chloride. Draw energy profiles for both chemical reactions.
Refer to the exothermic energy profile shown previously as an example. Are they the. Decomposition of calcium carbonateCalcium carbonate lime stone decomposes into calcium oxide quick lime and carbon dioxide when heated.
Quick lime is the major constituent of cement. Decomposition of potassium chlorateWhen heated strongly potassium chlorate decomposes into potassium chloride and oxygen. This reaction is used for the preparation of oxygen.
Calcium plays a vital role in the anatomy physiology and biochemistry of organisms and of the cell particularly in signal transduction pathways. More than 500 human proteins are known to bind or transport calciumThe skeleton acts as a major mineral storage site for the element and releases Ca2 ions into the bloodstream under controlled conditions. The treatment of potassium depletion particularly in the presence of cardiac disease renal disease or acidosis requires careful attention to acid-base balance volume status electrolytes including magnesium sodium chloride phosphate and calcium electrocardiograms and the clinical status of the patient.
Correct volume status acid-base balance and electrolyte deficits as appropriate. It is a chemical compound with the chemical formula CaCO 3. It is a white insoluble powder-like substance which occurs naturally in minerals chalk marble limestone calcite shells pearl etc.
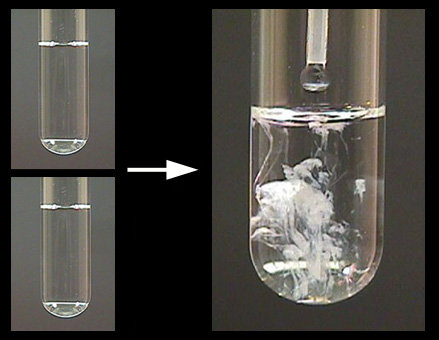
Medicinally it is used as an antacid or as a calcium supplement. Precipitation reactions can help determine the presence of various ions in solution. A solubility table can be used to predict precipitation reactions.
The process of an insoluble salt forming from its aqueous ions and falling out of solution. A method or writing a precipitation reaction without spectator ions. Precipitation refers to a chemical.
The normal method of producing acetylene was from calcium carbide. The high-energy requirement for carbide production was a serious drawback to the continuing mass production of vinyl chloride by this method 21. However as ethylene became more plentiful in the early 50s commercial processes were developed to produce vinyl chloride from chlorine and ethylene via EDC namely the.
Keep yourself from balancing the reaction until the 2 reactions are finished 10. On line 3You have to add coefficients to the ions and the compounds using the reaction form line 2. Take the subscript for the ion if it has one and multiply it by the coefficient for the compound just copy the coefficient from the previous reaction 11.
Ignore all spectator ions on line.