This reaction produces acetylene gas C 2 H 2 which burns a clean white flameAcetylene gas was originally discovered by Edmund Davy in 1836 but commercial production of calcium carbide only became feasible due to the efforts of Thomas Wilson in 1894. Calcium carbide CaC 2 reacts with water to form calcium hydroxide CaOH 2 and acetylene gas C 2 H 2.

Make sure a Type D fire extinguisher is available.
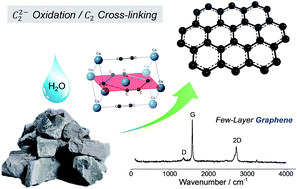
Calcium carbide and water reaction. The reaction of calcium carbide with water producing acetylene and calcium hydroxide was discovered by Friedrich Wöhler in 1862. CaC 2 2H 2 O C 2 H 2 CaOH 2 aq This reaction was the basis of the industrial manufacture of acetylene and is the major industrial use of calcium carbide. Today acetylene is mainly manufactured by the partial combustion of methane or appears as a side.
Calcium Ca and water Calcium and water. Reaction mechanisms environmental impact and health effects. Calcium occurs in water naturally.
Seawater contains approximately 400 ppm calcium. One of the main reasons for the abundance of calcium in water is its natural occurrence in the earths crust. Calcium is also a constituent of coral.
Rivers generally contain 1-2 ppm calcium but in lime. Transition metal carbides are not saline carbides but their reaction with water is very slow and is usually neglected. Alkali metals alkaline earth metals and lanthanoid metals form acetylides for example sodium carbide Na 2 C 2 calcium carbide CaC 2 and LaC 2.
Lanthanides also form carbides sesquicarbides see below with formula M 2 C 3. Metals from group 11 also tend. Carbide lamps are powered by the reaction of calcium carbide CaC 2 with water H 2 O.
This reaction produces acetylene gas C 2 H 2 which burns a clean white flameAcetylene gas was originally discovered by Edmund Davy in 1836 but commercial production of calcium carbide only became feasible due to the efforts of Thomas Wilson in 1894. Answer 1 of 6. CaCO3 H2O CO2 CaOH2 CaCO3 H2O CaO H2CO3 CaCO3 H2O CaOH2 H2CO3.
Solid calcium chloride is the solid left after the water evaporates. Calcium chloride is highly soluble in water because of its high solubility it is used to obtain solutions having relatively high densities. For example densities as high as 1430kgm 3 are achieved at 208 o C and 1570kgm 3 at 808 o C.
The oil and gas drilling industries frequently exploit these. Water has 4 regions of electron density around the central oxygen atom 2 bonds and 2 lone pairs. These are arranged in a tetrahedral shape.
The resulting molecular shape is bent with an H-O-H angle of 1045. The Use of Calcium Carbide in the Synthesis of 1-Monosubstituted Aryl 123-Triazole via Click Chemistry Y. Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction in Water Using Cyclodextrin as a Phase Transfer Catalyst J-A.
Chem 2012 77 4117-4122. In the presence of a readily accessible nanosized CuFe bimetallic system Cu. Calcium carbide CaC 2 reacts with water to form calcium hydroxide CaOH 2 and acetylene gas C 2 H 2.
When potassium chlorate KClO 3 is heated it decomposes to form KCl and oxygen gas O 2. C 6 H 6 combusts in air. C 5 H 12 O combusts in air.
Given the following reaction. Na 2 S 2 O 3 AgBr NaBr Na 3 AgS 2 O 3 2 a. The formed stable titanium carbide contains 14 C and the other radionuclides incorporated into the corundum and titanium carbide lattice.
Additional confining additives can be added to the reaction mixture for example zirconium which build even more stable crystalline phases with selected radionuclides such as uranium and plutonium. Furthermore additives are used to improve the final. Clinical efficacy of a sealer-based obturation using calcium silicate sealers.
A randomized clinical trial. Worldwide assessment of the mandibular first molar second distal root and root canal a cross-sectional study with meta-analysis. Martins et al.
Fenton-like reaction which produces highly toxic OH from the reaction between H 2 O 2 and ferrous ion Fe 2 has been broadly employed to induce cancer cell death Fig. This reaction forms both titanium oxide and highly flammable hydrogen gas according to the following reaction mechanism. Ti s 2H 2 O g - TiO 2 s 2H 2 g Some titanium compounds undergo hydrolysis reactions in water for example titanium chloride.
The Hazard fields include special hazard alerts air and water. Oxide silver perchloratecarbon tetrachloride mixture sodium hydroxide uraniumIV phosphide vinyl acetate calcium carbide rubidium carbide cesium acetylide rubidium acetylide magnesium boride mercuryII sulfate Lewis. Undergoes a very energetic reaction with calcium phosphide Mellor 88411946-1947.
GAS PRODUCTION METHODS Commercial instrument are also available that utilize specific reaction between chemical reagents and water that lead to the production of a gas. For example when a food sample is mixed with powered calcium carbide a amount of acetylene gas produced is related to the moisture content. CaC2 2H20 C2H2 gas Ca oH2 The amount of moisture produced can be.
Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature. Carbon monoxide CO is the other product of this reaction.
Metal hydrides and pyrophorics air or water reactive Examples. Sodium borohydride calcium hydride lithium aluminum hydride. Most metal hydrides react violently with water.
Make sure a Type D fire extinguisher is available. Contact EHS Fire Life Safety 858 822-5706 or 534-3531 if you have questions about fire extinguishers. An Explosive Chemical Reaction.
The reaction that takes place inside the pumpkin uses calcium carbide to generate a very small amount of acetylene gas. When the gas is ignited a tremendous amount of energy is released which pushes the pieces of the previously carved pumpkin out. Acetylene gas C 2 H 2 is very explosive and unstable.
It is a hydrocarbon and is the simplest alkyne. It is also. The presence of pollutants in aqueous solution particularly from hazardous heavy metals and metalloids is an important environmental and social problem.
Cooled in open air or in a circular cooler with water sprays or mechanical fans. The cooled sinter is crushed and screened for a final time then the fines are recycled and the product is sent to be charged to the blast furnaces. Generally 23 Mg 25 tons of raw materials including water and fuel are required to produce 09 Mg 1 ton of product sinter.
12512 Iron Production - Iron is. Water methods soften the dirt or soiling material and rinse the deposits. Tergitol by Union Carbide and Triton by Rohm Haas Thus the addition of a non-ionic detergent or surfactant to a low- or medium-pressure water wash can be a useful aid in the cleaning process.
A non-ionic detergent unlike most household detergents does not leave a solid visible residue on the masonry. Reagents 7 1 Deiomzed distilled water Prepare by passing distilled water through a mixed bed of cation and amon exchange resins Use deiomzed distilled water for the preparation of all reagents calibration standards and as dilution water 7 2 Nitric acid cone If metal impurities are found to be present distill reagent grade nitric acid m a borosilicate glass distillation apparatus or use a.