
If the formula is given write down the name and if the name is given write down the formula. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
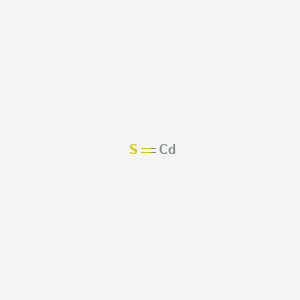
Sulfur dioxide SO2_ 2.
Cadmium sulfide formula. Sulfide British English also sulphide is an inorganic anion of sulfur with the chemical formula S 2 or a compound containing one or more S 2 ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds eg.
Lead sulfide and dimethyl sulfide. Hydrogen sulfide H 2 S and bisulfide SH are the conjugate. Cadmium sulfide Cadmium telluride.
Zinc selenide MercuryII selenide. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. What is Infobox references.
Cadmium selenide is an inorganic compound with the formula Cd Se. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. OptixeXpert is our new website that aims at connecting optical professionals with employers looking to outsource optics related work.
Sign up and fill out a short form to start offering your service. The composition of the sulfide minerals can be expressed with the general chemical formula A m S n in which A is a metal S is sulfur and m and n are integers giving A 2 S AS A 3 S 4 and AS 2 stoichiometries. The metals that occur most commonly in sulfides are iron copper nickel lead cobalt silver and zinc though about fifteen others enter sulfide structures.
Read More on This. Section D Write the formula for the ionic compounds containing transition metals BE CAREFUL TRANSITION METALS MAY HAVE ROMAN NUMERALS and NICKNAMES 1. Cadmium sulfide CdS 2.
Zinc iodide ZnI2 3. Iron III oxide Fe2O3 4. Lead II chloride PbCl2 5.
Magnesium nitride Mg3N2 6. Iron II fluoride FeF2 7. Copper II bromide CuBr2 8.
Silver nitride Ag3N 9. Lead IV oxide PbO2 10. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
An ionic compound is composed of a metal and a non-metal. Sodium chloride NaCl and magnesium oxide MgO. The transfer of electrons between metals and non-metals produces charged particles called ions.
Metals lose electrons to produce positve ions called cations. Na Mg 2 Non. Cd 3 AsO 4 2.
CdC 2 O 4 3H 2 O. Cd 3 PO 4 2. Chemical formula plays an important role in understanding different concepts of chemistry.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Chemical formulas can be.
Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice. You should complete this by Sunday.
Use the stock form for the transition metals. Give the formula for the following. Sulfur dioxide SO2_ 2.
Sodium thiosulfate Na2S2O3_ 3. If the formula is given write down the name and if the name is given write down the formula. Use these pages as a study guide.
As a general rule you should know the names and symbols for elements 1-36. In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently. Synthesis and characterizations of catalysts.
We had an interest in indium sulfide as a catalyst because S-doped In was shown by Wang and co-workers to. The positive charge more protons versus electrons for a cation is shown by a number and plus sign after the formula. If theres just a plus sign it means the charge is plus 1.
Review some examples of cations or positive ions. Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2 calcium Ca 2 cesium Cs chromium III Cr 3 cobalt Co 2. In a molecular formula it states the total number of atoms of each element in a molecule.
For example the molecular formula of glucose is C_6H_12O_6 and we do not simplify it into CH_2O. And for each compound they all have a molecular formula but some can be similar and those are called isomers which are common in organic chemistry. Melting Point C Physical Form.
Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. CH 3 2 CC 6 H 4 OH 2. Page 1 of 1.
Page 1 of 1. Support Customer Support Contact Us FAQ Safety Data Sheets SDS Certificates COACOO Quality Regulatory Calculators Apps Webinars.