0 degree Celsius C. Get 247 customer support help when you place a homework help service order with us.

Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction.
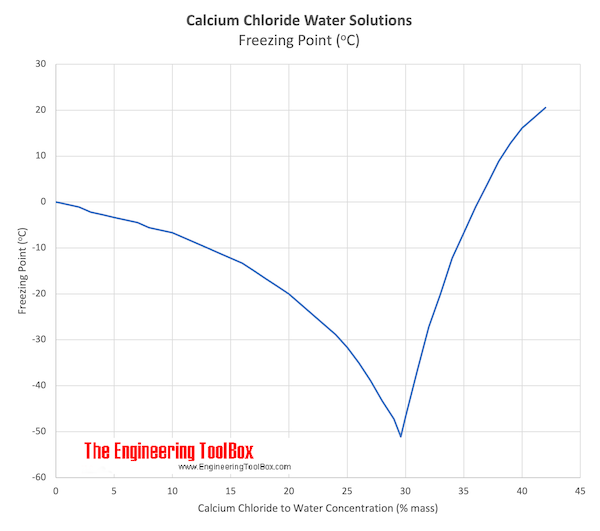
Cacl2 solubility in water at 20 degrees celsius. The solubility of KI is 50 g in 100 g of H2O at 20 C. If 110 grams of KI are added to 200 grams of H2O A all of the KI will dissolve. B the solution will freeze.
C the solution will start boiling. D a saturated solution will form. E the solution will be unsaturated.
The graph shows the solubility of several different salts in water across a range of temperatures. According to the graph which salt can dissolve at a concentration of about 60 g100 cm3 of water at 40 degrees Celsius. Solubility data for lead nitrate P b N O 3 2 in water at different temperatures are shown below.
Temperature C Solubility of lead nitrate gL of solution 20 560 40 750 60 950 80 1150. Fraction of mass of water due to neutrons 818 g. Does the solubility of a substance change with temperature.
Explain with the help of an example. Solubility is the ability of a solute to get dissolved in 100g solvent. Solubility of a given solute to dissolve in specific solvent depends on the temperature.
With an increase in. A 5075-g sample of water at 756 degrees C is added to a 16454-g sample of water at 241 degrees C in a constant-pressure calorimeter. Compute the heat lost by the high temperature sample of wate.
C3h8o2 molar mass email protected. C3h8o2 molar mass email protected. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. The freezing point depression constant for water Kf is 186 degrees Celsius per mole. If 20 moles of a substance are dissolved in 20 liters of solution what is the molarity of this solution.
How do you define the concentration units mass percent and molarity. What is the molarity of 100 g of KCI in 0500 L of solution. What volume of 0123 M NaOH in milliliters contains 250 g NaOH.
Water addition during the extraction phase varies from 0 up to a maximum of 10 at 20C. DMT at Carbatec - The Home of Woodworking. Heat up a normal pot of water to about 70 degrees Celsius.
They filter out the dust and keep your vacuum clean. Fill the bowl 34 with your smoking blend pack it down well we want a good bed of matter to catch every bit of melted dmt. Quantitative Chemical Analysis 7E Daniel C.
Chapter 7 Water Solution Solubility and Hydrogen 71 Open-hearth Process for Making Special Type of Steel 87 Non metals 89 Nitrogen 812 Phosphorous 817 Oxygen 819 Experiment I 820 Sulphur 821 Chlorine 828 Practice Questions 830 Hints and Explanation 836 Chapter 9 Organic Chemistry 91. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant.
Dates Lab Page Aug. 24-27 Separation of Mixture 16 Aug. 31-Sept3 Metric Estimates and Volume of Water Drop 21 Sept8-10 Density and Reliability of Data 28 Sept.
14-17 Solubility. Week 1 of zinc chloride 32 and 39 Sept. 21-24 Zinc Chloride Week 2 38 Sept.
1 Types of Reactions 41 Oct. 5-7 Writing Chemical Reaction Equations 49 Oct. If the 20 degC rate constant k is 02day and 9 105 find the correct BOD5 of the waste water.
190 mgL 19200 mL of Genesee river water was collected from just below the brewery Sample A 2 mL of river water diluted to ml aerated and seeded. The dissolved oxygen content was 76 mgL initially. After 5 days the dissolved oxygen cont had dropped to.
The melting points and boiling points of water at 1 atmosphere pressure. On the Celsius scale these points correspond to 0 C and 100 C respectively. 217 a b c 218.
The digits that are significant figures in a quantity are those that are known measured with certainty plus the last digit which contains some uncertainty. How many degrees Celsius is 100 degrees Farenheit. The temperature known as room temperature is nearest to A.
0 degree Celsius C. 20 degree Celsius B. 60 degree Celsius D.
100 degree Celsius 7. The volume of a gas is 50ml when the pressure is 1520 mm and the temperature is 0o C. The volume becomes 25 ml.
Then 10ml solution containing 1 g ion litre of Fe2. Fe3 togetherfreefromCI ions or other ions which interfere in KMn04 titration was diluted 10 fold with water. 20 ml of this diluted.
012 atm 200 L 122 L 0197 atm c If all the water remained in the gas phase the partial pressure of water at a total pressure of 200 atm would be PH 2O Ptotal mol fraction H 2 O 200 atm 012 240 atm However the partial pressure of water cannot be greater than 0197 atm and the excess will condense. The fraction that condenses is given by 240 atm 0197 atm fraction. Free essays homework help flashcards research papers book reports term papers history science politics.
Get 247 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. We always make sure that writers follow all your instructions precisely.
You can choose your academic level. High school collegeuniversity masters or pHD and we will assign you a writer who can satisfactorily meet your professors expectations.