Distilled water is a product of the distillation process. Calcium Carbonate Hydrogen Chloride Calcium Chloride Water Carbon Dioxide.

Distilled water is a product of the distillation process.
Cacl2 and water. E509 Cacl2 Calcium Dichloride Calcium2 Chloride CalciumIi Chloride Calcium Chloride Anhydrous CaCl2 Molar Mass CaCl2 Oxidation Number Water - H 2 O Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. Water base which includes alcohol-water mixtures and low strength acids make up the majority of treating fluids. The common chemicals added to these fluids are polymers for viscosity development crosslinkers for viscosity enhancement pH control chemicals gel breakers for polymer degradation following the treatment surfactants clay stabilizers alcohol bactericides fluid loss additives.
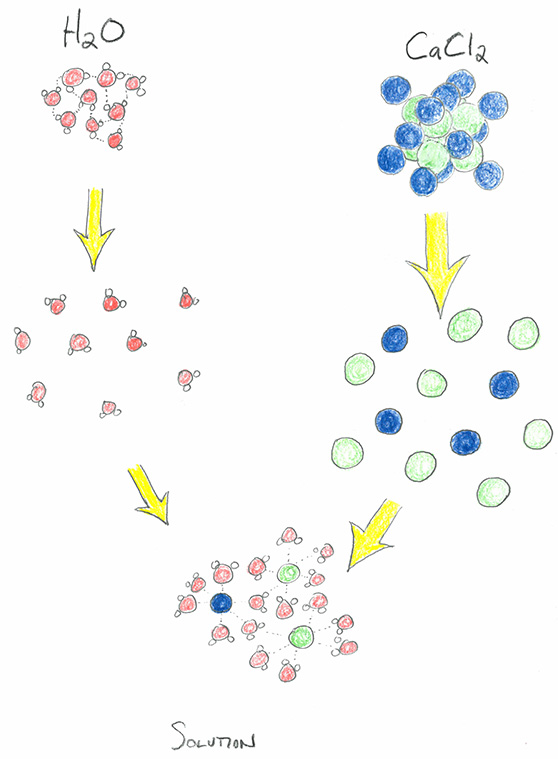
In this video we will describe the equation CaCl2 H2O and write what happens when CaCl2 is dissolved in waterWhen CaCl2 is dissolved in H2O water it wil. We would like to show you a description here but the site wont allow us. Calcium Chloride - CaCl2.
What is Calcium Chloride CaCl 2. CaCl 2 is an ionic compound with chemical name Calcium Chloride. It is also called Calcium chloride anhydrous or Calcium dichloride.
It is an ionic compound of chlorine and calcium. At room temperature it is a crystalline solid white in colour. It is highly soluble in water and hence is hygroscopic in nature.
It is odourless and has. To prepare 1000 mL of a 01 molL solution of AlCl3 we have to dissolve 241433 g of AlCl36H2O 96 purity in deionized or distilled water. After the solid is completely dissolved dilute the solution to a final volume with deionized distilled water.
We will need to dilute 1372 mL of 73 AlCl36H2O to a final volume with deionized. Balance The Equation Caco3 Hcl Cacl2 H2o Co2. CaCO 3 HCl CaCl 2 H 2 O CO 2.
CaCO 3 2HCl CaCl 2 H 2 O CO 2. Calcium Carbonate Hydrogen Chloride Calcium Chloride Water Carbon Dioxide. Calcium carbonate is not very soluble in water.
This is an acid-base reaction neutralization. CaCO 3 is a base HCl is an acid. Heres what I got.
Interestingly enough Im not getting 00341 wv either. Start by calculating the percent composition of chlorine Cl in calcium chloride This will help you calculate the mass of chloride anions Cl- present in your sample. To do that use the molar mass of calcium chloride the molar mass of elemental chlorine and the fact that 1 mole of calcium.
It has been discovered experimentally that for particular types of water there is an approximate relationship. In water with a higher proportion of sodium chloride to get to ppm just multiply the µScm reading by 05. For most other water for example in hydroponics.
- Buffers Chemicals Gloves Transfection Reagent Genotyping Reagents PCR Reagents and Supplies siRNA and microRNA Enzymes Pipette Tips Bacteria Culture Supplies Slides Box and Mailer Water Purification Parts Bottles. Water and CaCl2 were used as controls. Nitrogenous compounds increased percent germination level and rate in three of the species studied.
High pH negatively affected the germination rate of seeds from most species but had no effect on the per cent germination of any of the species. The higher concentration of the nutritious solutions affected negatively the germination level and rate. What is Distilled Water.
Distilled water is a product of the distillation process. In the distillation process water undergoes boiling to turn into steamThis will be captured and cooled that becomes the distilled waterIt leaves behind all the contaminants including inorganic minerals chemicals and metals. Soil pH in water Distilled water is used in place of 001M calcium chloride and results are expressed as pHw.
The pHCaCl2 test is the more accurate of the two pH tests as it reflects what the plant experiences in the soil. The values of pHCaCl2 are normally lower than pHw by 05 to 09. A useful but not consistently accurate.
The project will also produce the following useful by-products. Hydrochloric Acid HCl Calcium Chloride CaCl2 and Sodium Hypochlorite NaOCl. Learn More Our Products.
Dissolve the reagent salts in 800 ml distilled water. Adjust the pH to the desired level with hydrochloric acid. Usually this is 74 or 72.
Use a pH meter to measure the pH not pH paper or other imprecise technique. Add distilled water to achieve a final volume of 1 liter. Sterilization and Storage of PBS.
Claudin-2 a component of the tight junction forms a paracellular water channel. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function.
By Susanne Krug and I. Altered paracellular cation. The CaCl2CaBr2 halide mixture was revealed to form solid solutions in any molar contents.
Ammonia absorption abruptly increased at some pressure forming the ammine complex and the step. The geometry of water molecules and the bond lengths of MIBC were remained unchanged by means of LINCS algorithms. 2SO4 and Na2CO3 Relative to CaCl2 and NaCl at the AirAqueous Interface Revealed by Heterodyne Detected Phase-Sensitive Sum Frequency.
Lett 2 2011 p. CrossRef View Record in Scopus Google Scholar GPW. Soil water-holding capacity mediates hydraulic and hormonal signals of near-isohydric and near-anisohydric Vitis cultivars in potted grapevines.
Drought-induced changes in development and function of grapevine Vitis spp organs and in their hydraulic and non-hydraulic interactions at the whole-plant level. A physiological and molecular update. By Claudio Lovisolo.
Process Water Other Tap water m3 032 Process Water Other Water m3 003 Process Water Other Water decarb. M3 001 Process Water Other Water deion. M3 080 Process Water Other Water solf.
M3 002 Reagent Chemical AlOH3 Aluminium hydroxide AlOH3 kg 066 Reagent Chemical Al2SO43 Aluminium sulphate powder Al2SO43 kg 050 Reagent Chemical Al2O3 Aluminium oxide Al2O3 kg. Collection of water and sediment samples is the first step of microplastics sampling methodologies. The choice between sampled medium is dependent on available equipment but also the objective of the work.
For instance sampling the water column may be the most adequate medium if the objective is to determine the exposure of pelagic organisms. However microplastic distribution is. Volumetric water content at -10 kPa of volume.
Volumetric water content at -33 kPa of volume. Volumetric water content at -1500 kPa of volume. The amount of phosphorous using the Bray1 method.
The amount of phosphorous by Olsen. Water NaCl NaF MgNO32 Na2O C18H35NaO2 NaH MgO C6H12O6 C2H3NaO2 NaClO C2H45 SO4 KI H2O COMPOUNDS CONT 6 Image Compound Name Chemical Formula Mercuric Chloride Calcium Chloride Potassium Chloride Cerium Chloride Tungsten Chloride HgCl2 CaCl2 KCl CeCl3 WCl6 COMPOUNDS CONT CHLORIDES. LAB TABLE 7 Use the Lab Table by right clicking on it just like a.
0543g of CaCl2 was dissolved in enough water to make a 500ml solution. What is the concentration of Cl- in wv. A solution of HCl in water has a density of 11 gmL.
What is the mass of HCl in the solution if 150 mL of the solution contains 30. CaCl2 16g water 100g dry ice -10 CaCl2 27g water 100g dry ice -20 CaCl2 36g water 100g dry ice -30 CaCl2 42g water 100g dry ice -40 CaCl2 48g water 100g dry ice -50 続実験を安全に行うために化学同人第6章冷却図1のグラフより読み取った値 シリカゲルカラムクロマトグラフィー 参考文献 W. Stille et al.