Water evaporates the dissolved salts remain resulting in a solu - tion with a higher concentration of salt. If we add a smaller amount of water to a.
The natural sources are surrounding rock or soil or seawater intrusion in coastal areas.
Cacl dissolved in water. Chemicals added to react with dissolved oxygen sulphate hydrazine etc and chemicals used to prevent scale and corrosion in the feed-water system polyphosphates organics etc should be fed in the feed-water system as continuously as possible. Chemicals used to prevent condensate system corrosion may be fed directly to the steam or into the feed-water system depending on the specific. 0543g of CaCl2 was dissolved in enough water to make a 500ml solution.
What is the concentration of Cl- in wv. What is the concentration of Cl- in wv. Ok so I calculated the concentration to be 962x10-3 and then multiplied this by 2 since there are 2 chlorine ions.
CaCl 2 is an ionic compound with chemical name Calcium Chloride. It is also called Calcium chloride anhydrous or Calcium dichloride. It is an ionic compound of chlorine and calcium.
At room temperature it is a crystalline solid white in colour. It is highly soluble in water and hence is hygroscopic in nature. It is odourless and has a very high enthalpy change of solution.
This compound is. Calcium chloride is an inorganic compound a salt with the chemical formula CaCl 2It is a white colored crystalline solid at room temperature and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Calcium chloride is commonly encountered as a hydrated solid with generic formula CaCl 2 H 2 O x where x 0 1 2 4 and 6. Determine the molarity when 555g of CaCl 2 are dissolved to make 250mL of solution. 1st convert to moles 2nd convert to liters 3rd plug into the molarity equation.
555g CaCl 2 1mol111g 0500mol CaCl 2. 10 What is the concentration in ppm if 0808 grams of CaCl 2 is dissolved in 2500 ml of water. 11 What is the concentration of chloride in a solution made with 0808 grams of CaCl 2 and 2500 ml of water.
Give your answer in ppm. Water escaping as steam for example from a boiler of heating system or steam engine leaves behind any dissolved materials leading to mineral deposits known as boiler scale. A boiling water distiller.
Boiling tank on top and holding tank on the bottom. Low-volume humidifiers such as cigar humidors can use distilled water to avoid mineral deposits. Certain biological applications require.
The main cause of water hardness is dissolved salts of calcium and magnesium. Moreover the other ions like Strontium Iron Barium and Manganese also contribute of water hardness. Traditional it is measured by the amount of soap that is required to produce leather.
The EDTA method is more accurate and more rapid. Mn Na 2 H 2 EDTA M-EDTA 4-n- 2Na 2H There are two types. For 100 ml stock solution dissolve 1470 g CaCl2-2H2O in 945 ml water.
Autoclave for 15 min at 121C. Biotin 1 mgml. Add small aliquots of 1N NaOH until the biotin has dissolved.
Add water to a final volume of 50 ml. Sterilize the solution over a 022-µM filter. Prepare 1 ml aliquots and store at -20C.
Thiamin 1 mgml thiamin-HCl 1mgml 50 mg50 ml For 50 ml stock solution. CaCl 2 Buffers Preparation 1M CaCl 2 stock solution 10x working concentration Weigh out 111g of anhydrous CaCl 2 Add to 80mL of dH2O Mix solution until CaCl 2 is fully dissolved Top up to 100mL Filter sterilize through a 022μm pore 01M CaCl 2 working solution Add 10mL of 1M CaCl 2 to 90mL of dH2O for a 110. Analyze gel images from any source.
Use your digital camera smartphone or gel doc system to obtain images. GelAnalyzer will take care of the rest. For the MW of CaCl 2 add the atomic mass of Ca 4001 to that of two Cl 2 x 3545 to get 11091 gmole.
Therefore a 1M solution of CaCl 2 consists of 11091 g of CaCl 2 dissolved in enough water to make one liter of solution. Once the molecular weight of the solute is known the weight of chemical to dissolve in a solution for a molar solution less than 1M is calculated by the. And dead air and water.
The water component is where pH is measured where dissolved chemicals cause the soil to be acidic or alkaline. Soil acidity and alkalinity are measured in units of pH. The pH scale is from 0 most acid to 14 most alkaline and a pH of 7 is neutral.
Understanding LEAFLET NO2 Soil pH The pH of a soil will change over time influenced by factors including parent. OCa NaCl ONa CaCl 2 Depending on the level of regeneration the hardness can be reduced to less than 1 ppm but typically the target is simply less than 171 ppm or one grain of hardness per gallon gpg. One grain is equal to 171 ppm of hardness expressed as calcium carbonate.
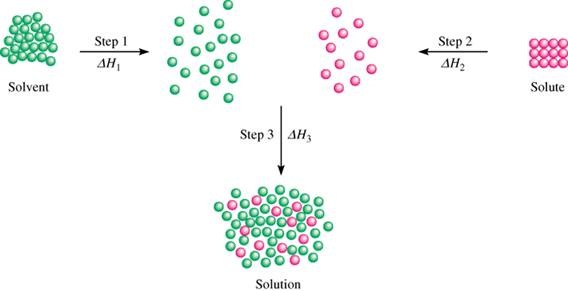
Softeners do not reduce the total dissolved solids TDS or dissolved mineral content of water. Amount of water to ensure that the dissolved ions are well separated and do not interact with one another. NaCl s aq Na aq Cl-aq Enthalpy change of formation The standard enthalpy change of formation of a compound is the energy transferred when 1 mole of the compound is formed from its elements under standard conditions 298K and 100kpa all reactants and products being in.
After the solid is completely dissolved dilute the solution to a final volume with deionized distilled water. We will need to dilute 1372 mL of 73 AlCl36H2O to a final volume with deionized distilled water. Transfer the prepared solution to a clean dry storage bottle and label it.
Never store solutions in a volumetric flask. All chemicals that you are unfamiliar with should be. Testing for Water Hardness Background Info.
Hard water has a lot of minerals Calcium and Magnesium mainly dissolved in it. Hard water does not mix well with soaps. Because of this you can test for the hardness of water by looking at the amount of bubbles produced when you mix water and soap.
Hard water is water containing high concentrations of dissolved minerals usually calcium or magnesium carbonates CaCO 3 or MgCO 3 chlorides CaCl 2 or MgCl 2 or sulphates CaSO 4 or MgSO 4. The hardness of water depends on its source. Groundwater that has been in contact with porous rocks containing deposits of minerals like limestone or dolomite will be very hard while water.
The most common salts are NaCl KCl MgCl 2 and CaCl 2. They are extremely soluble in water. The Sources of chloride in water may be natural or human beings.
The natural sources are surrounding rock or soil or seawater intrusion in coastal areas. Whereas various human sources are fertilizers road salting wastewater from industries animal feeds septic tank effluents etc. CaCl 2 Na 2 CO 3 CaCO 3 2NaCl.
Magnesium oxide is the preferred chemical because it does not increase the dissolved solids concentration of the water. Good sludge contact enhances silica reduction. To ensure optimum contact sludge is frequently recirculated back to the inlet of the unit.
Cold or warm process softening is not as effective as hot process softening. Various separation media have been used including water or water solutions of known density alcohol NaCl CaCl 2 or ZnCl 2. As shown in the following table the densities of common plastics differ sufficiently to permit them to be discriminated in this fashion.
The cylindroconical cyclone device shown on the right provides a continuous feed procedure in which the material to be separated. Concentration refers to the amount of solute that is dissolved in a solvent. We normally think of a solute as a solid that is added to a solvent eg adding table salt to water but the solute could easily exist in another phase.
For example if we add a small amount of ethanol to water then the ethanol is the solute and the water is the solvent. If we add a smaller amount of water to a. Formula of calcium chloride is CaCl2.
The dry residual is also called Total Dissolved Solids and abbreviated as TDS. Rohm and Haas Ion Exchange Ion exchange introduction 3 FD Sep 2008 Ion Exchange Impurities in water Water as we have seen contains small amounts of foreign substances. In many cases these substances cause no problem.
Drinking water containing some salinity is much. 2 CaOH 2 2 Cl 2 CaClO 2 CaCl 2 2 H 2 O Sodium Process. 2 CaOH 2 3 Cl 2 2 NaOH CaClO 2 CaCl 2 2 H 2 O 2 NaCl.
But how can this chemical be used to sterilize water. This chemical can be used for sterilizing water by Using 5 drops of bleach per each half gallon of water to be purified and allowing it to sit undisturbed for half an hour to make it safe for drinking. Water evaporates the dissolved salts remain resulting in a solu - tion with a higher concentration of salt.
The same process occurs in soils. Salts as well as other dis - solved substances begin to accu - mulate as water evaporates from the surface and as crops withdraw water. Units Terms and Sampling Numerous parameters are used to define irrigation water quality to assess.