Go to Molarity Problems 26-35. 117 States of Matter PhET Labdoc 19 kB.

Mass molarity x molar mass x volume of solution 280 mol L.
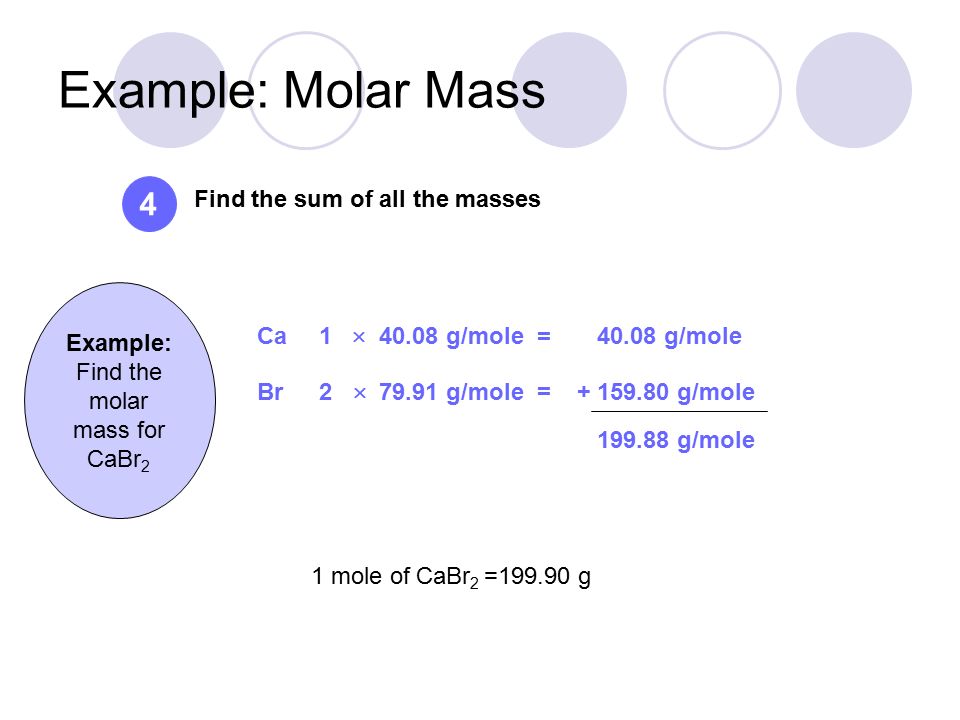
Cabr2 molar mass. Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. What mass in grams of AlI3 is consumed when 46 grams of HgI2 is produced.
A 275 b 69 c 1376 d 825 e 688 2 Given the following reaction. CaBr2 2 KOH —– CaOH2 2 KBr What mass in grams of CaBr2 is consumed when 96 g of CaOH2 is produced. A 173 b 52 c 86 d 155 e 259 3 Given the following reaction.
CaO SiO2 CaSiO3 2 CO2 CaSiO3 H2O SiO2 CaHCO32 CaSiO3 6 HF 3 H2O SiF4 CaF2 SiO2 CaOH2 CaSiO3 H2O SiO2 CaCO3 CaSiO3 CO2 CaSiO3 H2SO3 H2SiO3 CaSO3 CaBr2 Na2SiO3 2 NaBr CaSiO3 CaCl2 Na2SiO3 CaSiO3 2 NaCl K2SiO3 CaC2H3O22 CaSiO3 2 KCH3COO. MV mass molar mass x 100 L 200 g 199886 gmol x 0100 M When CaBr 2 ionizes two bromide ions are released for every one CaBr2 that dissolves. That leads to this.
Br- 0200 M. Go to Molarity Problems 1-10. Go to Molarity Problems 26-35.
Go to Molarity. Return to Solutions Menu. 19989 gmol anhydrous 23598 gmol dihydrate Appearance anhydrous is hygroscopic colorless crystals sharp saline taste Density.
730 C 1350 F. 1000 K Boiling point. 1815 C 3299 F.
2088 K anhydrous 810 C dihydrate Solubility in water. 125 g100 mL 0 C 143 g100 mL 20 C 312 g100 mL 100 C Solubility in alcohol. Agno3 SilverI Nitrate Nitric Acid SilverI Salt AgNO3 Molar Mass AgNO3 Oxidation Number.
Calcium Chloride - CaCl 2. E509 Cacl2 Calcium Dichloride Calcium2 Chloride CalciumIi Chloride Calcium Chloride Anhydrous CaCl2 Molar Mass CaCl2 Oxidation Number. Silver Chloride - AgCl.
Agcl Agcl SilverI Chloride AgCl Molar Mass Bond Polarity AgCl Oxidation Number. Molarity is the number of moles per liter of solution. Whereas number of moles is defines as mass divided by molar mass.
Since it is given that molarity is 280 M volume is 500 ml or 05 L and molar mass of KI is 166 gmol. Mass molarity x molar mass x volume of solution 280 mol L. In an aqueous solution NH3 turns to NH4OH and the molar mass is 3504grmol not 17 grmol.
So 1 M means 3504gr in a liter of water and because the solution concentration is 25 the amount of. It has a molar mass of 99 gmol. Analysis of a sample shows that it contains 243 carbon and 41 hydrogen.
What is its molecular formula. Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition.
2803 Mg 2160 Si 116 H and 4921 O. The molar mass for chrysotile is 5208 gmol. Polymers are large molecules.
Solucionario quimica de raymond chang 12 edicion. Solucionario quimica de raymond chang 12 edicion. Chemistry 12th Edition by Chang and Gold.
Calculate the molarity mass percent mole fraction and molality of ethanol _2 0. Elements of physics motion force and gravity forces worksheet 1 answer key. How many moles of sucrose are dissolved in 250 ml of solution if the solution concentration is 0 150 m.
117 States of Matter PhET Labdoc 19 kB. Ph virtual lab answer key Sep 02 2012 pH and pOH. Molar mass of KNO3 101.
2 with Azide was tested on trypticase soy agar for 24 hours 48 hours and 72 hours and was found to be negative for bacteria. May 29 2020 For 0. The The pH of a solution ranges from 1-14 1-6 are acidic 7 is neutral and 8-14 are basic.
Acidic - Introduction to pH - the acidic and basic alkaline definition. Can you explain this answer. Is cacl2 soluble in water email protected.
Combustion reactions chem worksheet 10 6 answers. Combustion reactions chem worksheet 10 6 answers. Since the mass is less than half the molar mass 4296 05 the number of formula units should be less than half Avogadros number 26 x I02360 x 1023 05.
A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass. Converting amu to grams. Free essays homework help flashcards research papers book reports term papers history science politics.
Example alculate the mass of a charged particle in CGS units if its charge is x coulomb and specific charge C is y coulombg. Solution The mass of the particle in CGS units is e g x g. E y m Example he isotopes of an element have mass numbers A A 1 A 2.
The ratio of abundance of these T isotopes is 3. Calculate the average atomic mass of the element solution Average atomic mass. The molar mass of a compound is the sum of molar masses of the elements it contains.
Pest control with gaseous pesticides. Being transported under the transportation of dangerous goodstdg regulations get access to this video and our entire Q a. Since the electronegativity of phosphorous and hydrogen is nearly same so the covalent bond is non-polar.
Compound NameLet us practice by naming the.