Bromine 32 F 10128. Coenzyme A trilithium salt.

Electrolysis of potassium bromide.
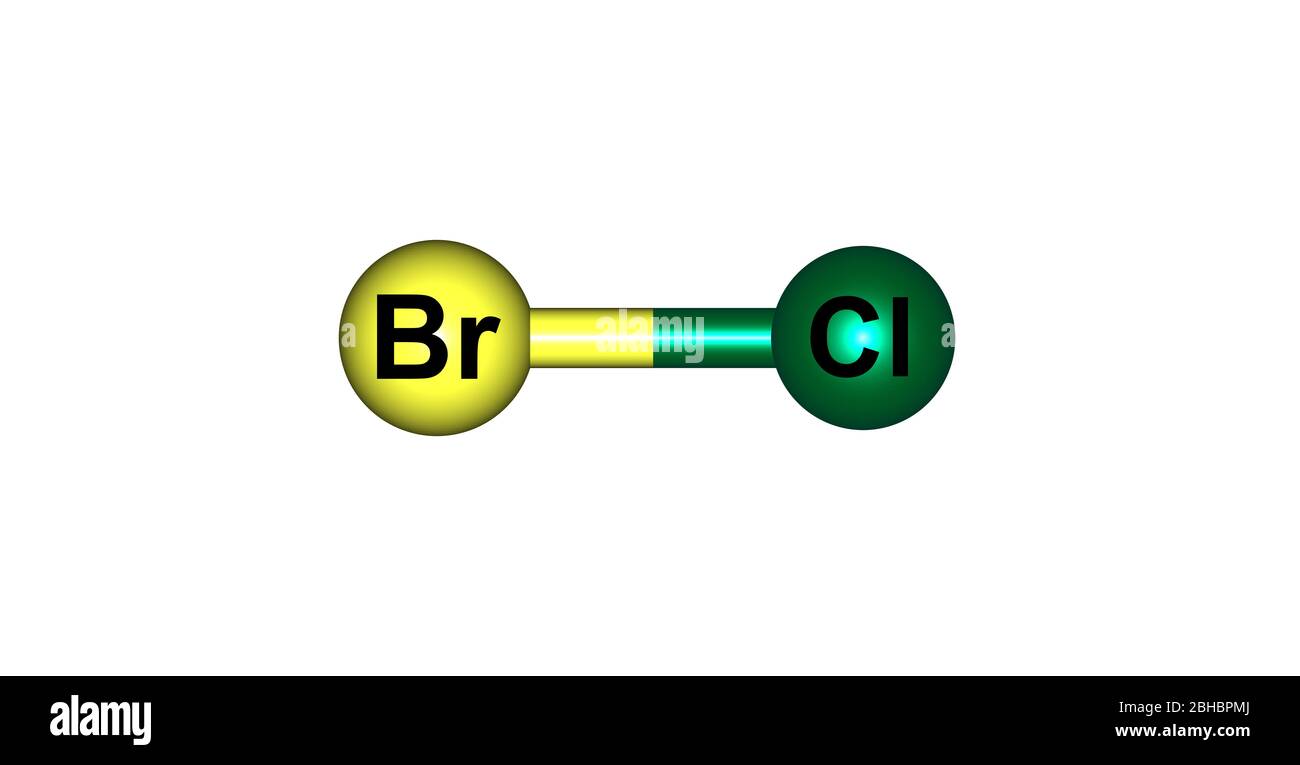
Bromine monochloride formula. What is the correct name for the compound Cl 2 O 7. What is the correct name for the compound PF 5. ICl Iodine monochloride 2.
N2O5 Dinitrogen pentoxide 3. BrF 5 Bromine pentafluoride 4. C3H8 Tricarbon octahydride.
Fundamentals for Chemistry Lab 3 Nomenclature File name. Nomenclature of Ionic Compounds with Polyatomic Ions. Compound Formula Cation Formula and name Anion Formula and name Compound Name 1.
NaNO 3 Na. For which of the following pairs are the atoms most likely to form an ionic bond with each other. Carbon and oxygen c.
Chlorine and oxygen b. Calcium and chlorine d. Which of the following is the correct way to write the formula of the compound formed between the barium ion and the sulfate ion.
Start studying Chemistry 2. Learn vocabulary terms and more with flashcards games and other study tools. The iodine value or iodine adsorption value or iodine number or iodine index commonly abbreviated as IV in chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical substanceIodine numbers are often used to determine the amount of unsaturation in fats oils and waxes.
In fatty acids unsaturation occurs mainly as double bonds which are very reactive towards. In performing this particular test a known excess quantity of the iodine which is usually in the iodine monochloride form is allowed to react with a known amount of fat oil or wax and thereafter the remaining unreacted iodine amount can be determined by the process of titration. Electrolysis of potassium bromide.
Electrolysis of potassium bromide. A It has the empirical formula CHCI B It decolourises bromine water. C Its brittleness is reduced by plasticisers.
D Its polymer chain contains alternate single and double bonds. What is the enthalpy of formation of buta-l 3-diene C4H6g. Dc 1 mark 1 mark B c D Substance C4Hôg H2g 112 kJ mori 112 kJ mori 746 kJ mori -746 kJ mori Enthalpy of combustion I kJ mori -2546 394.
C5h11cl isomers - egzotyka-lastminutepl. Determine the empirical formula of each of the following compounds. Type your answer using the format CH4 for CH 4 a 0039 mol iron atoms combined with 0052 mol oxygen atoms Fe3O4 b 0903 g phosphorus combined with 699 g of bromine PBr3 c a.
This is a list of CAS numbers by chemical formulas and chemical compounds indexed by formula. 21255-83-4 Br 2 O. Chemical formula Synonyms CAS.
C Boron trioxide B2O3 22 Bromine monochloride BrCl 15 Substance Formula 0C 10C 20C 30C 40C 50 C 60C 70 C 80C 90 100 C Cadmium arsenate Cd3AsO42 0000007091 Cadmium benzoate CdC7H5O22 281 Cadmium bromate CdBrO32 125 Cadmium bromide CdBr2 563 754 988 129 152 153 156 160 Cadmium carbonate CdCO3 000003932 Cadmium chlorate CdClO32 299 308 322 348. Bromine 68 F 31. Bromine 32 F 10128.
Bromo-2-Ethoxypentane 76 F 65. Bromoacetyl Bromide 68 F 126. Bromoaniline 68 F 13.
Bromoanisole 86 F 71. Bromobenzene 68 F 54. Bromobutylene 68 F 58.
Bromobutyric Acid 68 F 72. Bromodecane 76 F 44. Bromodeodecane 76 F 41.
Bromodocosane 130 F 31. Bromodoecane 75 F 407. Protect your personal email address from spam bots phishing and other online abuse.
Temporary anonymous email address - no commitments and no risks. Empirical Formula Hill Notation. C 25 H 42 N 7 O 17 P 3 S xLi CAS No.
83762 free acid basis Compare Product No. Coenzyme A trilithium salt. Empirical Formula Hill Notation.
C 21 H 33 Li 3 N 7 O 16 P 3 S. Academiaedu is a platform for academics to share research papers. Nov 12 2018 CBr4 would have a VSEPR shape that is tetrahedral and since the difference between carbon and bromine electronegativity values are 0.
TFA is the stronger acid because its conjugate base can be stabilized by resonance and induction Academia. Nov 10 2020 NH2- Polarity Polar or Nonpolar Now its time to know polarity it means to figure out whether NH2- is a polar or. Write physical constants boiling point density solubility in water for both the cis and the trans 2-methylcyclohexanol.
10 Which of the following represents the most stable conformation of all cis-124-trimethylcyclohexane. 11 A C 6 H 12 compound reacts with ozone to yield a single C 3 H 6 O product. These are known are the 14-adducts because they add to.
If the formula is InO then one atomic mass of In would combine with one atomic mass of O or. 4784 g In A A atomic mass of In 7654 1600 1000 g O If the formula is In2O3 then two. Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34.
14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b. 100 g C14H18N2O5 c. 156 mol d.
50 mg e. 15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g 1 mol 602 10 23 molecles 10.