
The Halogens The elements in Group 17 are. Let us learn about the physical and chemical properties of water.
So it displaces iodine in potassium iodide compounds.
Bromine chemical properties. Chemical properties of elements and compounds. Atomic number - Atomic mass - Electronegativity according to Pauling - Density - Melting point - Boiling point - Vanderwaals radius - Ionic radius - Isotopes - Electronic schell - Energy of first ionisation - Energy of second ionisation - Standard potential. Atomic number The atomic number indicates the number of protons within the core of an atom.
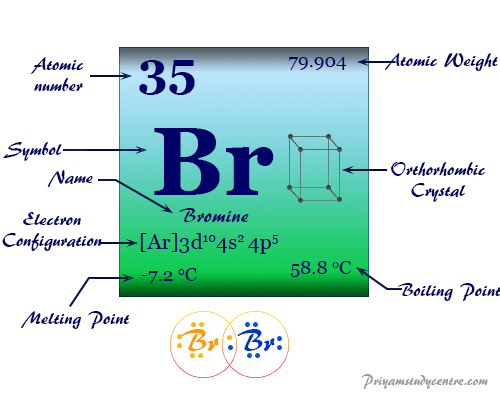
Atomic Number Protons Electrons and Neutrons in Bromine. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleusTotal number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. Physical and Chemical Properties of Group 17 Elements Group 17 Elements.
The Halogens The elements in Group 17 are. Fluorine Chlorine Bromine Iodine Astatine These elements are known as halogens. A Halogen is a Greek word which means salt-former.
B This is because halogens are reactive non-metals. They exist naturally in various mineral salts in. Bromine to produce metal bromide.
To predict the properties of rubidium caesium and francium. Rubidium caesium and francium are placed below potassium in Group 1 of the Periodic Table. Hence rubidium caesium and francium are expected to react with water oxygen chlorine or bromine in a similar way as potassium but these reactions are more vigorous more reactive than potassium.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.
Its properties are thus. Physical Chemical Properties of Tungsten. Tungsten is one of the important strategic resources.
Due to its excellent physical and chemical properties tungsten and its alloys are used to manufacture key armor-piercing components that attack various types of armored targets gyro inertial components for satellites and high-temperature anti-ablation components such as rockets combustion. The elements in group 1 are called the alkali metals. Group 1 is on the left-hand side of the periodic table.
The alkali metals share similar physical. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br.
Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Its properties are thus. Water is the chemical substance with chemical formula H 2 O one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Let us learn about the physical and chemical properties of water. A glance of earth taken from space will depict it blue. This blue colour is actually water the major part of.
Oxygenated solvents are produced through chemical reactions from olefins derived from oil or natural gas giving us the following sub-groups. Alcohols ketones esters ethers glycol ethers and glycol ether esters. The human body naturally produces ketones when it burns fat.
Halogenated solvents are solvents that contain a halogen such as chlorine bromine or iodine. Many people recognize. Chemical properties of vanadium - Health effects of vanadium - Environmental effects of vanadium.
Electronegativity according to Pauling. 61 gcm-3 at 20C. 0074 nm 3.
Electronic shell Ar 3d 3 4s 2. Example Bromine is more reactive than iodine. So it displaces iodine in potassium iodide compounds.
Reaction is given below 2KI Br 2 2KBr I 2. Properties of Metals. Gold aluminium iron and magnesium etc.
They show following properties Physical Properties of Metals. Metals can be hammered into thin sheets. It means they possess the property of malleability.
Chemical Properties of Zinc. 419527C 787149F 692677 K. 907C 1665F 1180 K.
Density g cm 3 7134. 6538 State at 20C. Electron configuration Ar 3d 10 4s 2.
ChemSpider is a free chemical. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like.
The Periodic Table of Elements ELEMENTS IN SAME COLUMN GROUP HAVE SIMILAR CHEMICAL PROPERTIES. The name of each element in brown is accompanied by its chemical symbol in red as well as its atomic number Z and its most common or most stable mass number A. Z atomic number number of protons in the nucleus number of electrons orbiting the nucleus.