
Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY. 310b Mass of CaCl2 containing chlorine 2m.
311a Mass of H in.
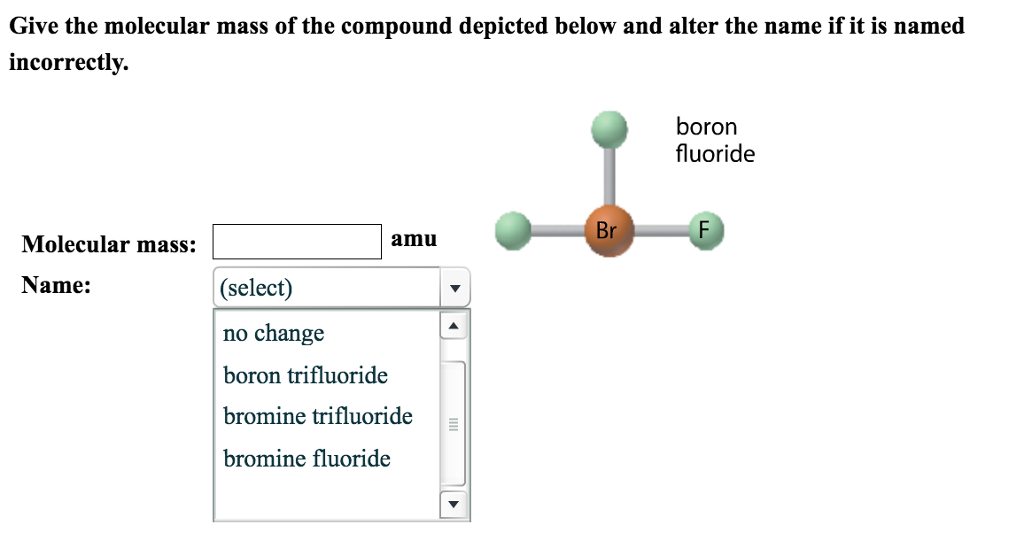
Boron triflouride molar mass. 6782 gmol anhydrous 103837 gmol dihydrate Appearance colorless gas anhydrous colorless liquid dihydrate Density. Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers. CsF BF 3 CsBF 4 O C 2 H 5 2 BF 3 BF 3 OC 2 H 5 2.
Tetrafluoroborate salts are commonly employed as non-coordinating anions. 307c Formula for boron triflouride 40s. 309 Molar Mass of Compounds 11m.
309a Molecular Mass of Al2Cr2O73 1m. 309b Mass of a sample of vitamin C 2m. 309c Moles of sucrose within a sugar cube 2m.
310 Mass percent composition 8m. 310a Mass percent of chromium 1m. 310b Mass of CaCl2 containing chlorine 2m.
311 Relationships from Chemical Formulas 11m. 311a Mass of H in. Nickel iii sulfide chemical formula.
The boron in BF3 is electron poor and has an empty orbital so it can accept a pair of electrons making it a Lewis acid. The octet Jun 16 2021 1. Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY.
Lewis electron dot structure calcium hydride. Since the iodine has 7 valence electrons out of which 3 electrons form sigma bond with F atoms and left with forming 2 lone pair.