Ethanol Absolute 200 Proof Molecular Biology Grade Fisher BioReagents. In 1982 the International Union of Pure and Applied Chemistry IUPAC0 defined the standard boiling point as the temperature of boiling under 1 bar of pressure.

It is a solvent for dyes nitrocellulose paints inks and resins.
Boiling of ethanol. Boiling Points of Water and Ethanol. Boiling points of liquids in literature are generally referenced to the sea level at an atmospheric pressure of 760 mm Hg. Boiling points change depending on altitude and atmospheric pressure changes.
Boiling Points for Water and Ethanol at Different Altitudes Atmospheric Pressures. Below is a short boiling point table with values for 4 different. Pour 10cm3 of ethanol into a 100cm3 round bottomed flask.
Put this into a water bath. Carefully add 10cm3 concentrated sulphuric acid and swirl. Wipe off the water from the flask with tissue paper.
Clamp the flask inside an empty 400cm3 beaker supported on a tripod and gauze. Fit a two-necked adapter to the round bottomed flask. Place a separating funnel into the adapter so that liquid can run.
2-2-Ethoxyethoxyethanol also known under many trade names is the organic compound with the formula CH 3 CH 2 OCH 2 CH 2 OCH 2 CH 2 OH. It is a colorless liquid. It is a popular solvent for commercial applications.
It is produced by the ethoxylation of ethanol. It is a solvent for dyes nitrocellulose paints inks and resins. Ethanol intended for industrial use is usually denatured rendered unfit to drink typically with methanol benzene or kerosene.
Pure ethanol is a colourless flammable liquid boiling point 785 C 1733 F with an agreeable ethereal odour and a burning taste. Ethanol is toxic affecting the central nervous system. Moderate amounts relax.
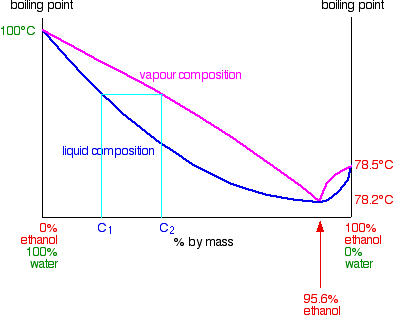
Boiling component of the original feed. If energy was cheap and the ethanol-water system was ideal then this rather simple distillation system would suffice for the sep-aration of the beer feed into a relatively pure ethanol overhead product and a bottoms product of stillage cleanly stripped of its ethanol content. Unfortunately the ethanol.
Ethanol is a primary alcohol that is ethane in which one of the hydrogens is substituted by a hydroxy group. It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. Solvent boiling points acquired from materials published online by Honeywell Burdick and Jackson University of Oxford University of Louisville Michigan State University and IPS INCHEM and are inteded for use with the BrandTech Scientific Vacuum Pump Selection Guide only.
Solvent listing does not necessarily imply chemical or physical compatibility with. The boiling point of the alcohol ethanol is 7829 C compared to 69 C for the hydrocarbon hexane and 346 C for diethyl ether. Simple alcohols are found widely in nature.
Ethanol is the most prominent because it is the product of fermentation a major energy-producing pathway. The other simple alcohols are formed in only trace amounts. More complex alcohols however.
Sometimes boiling point is defined by the pressure at which the measurement was taken. In 1982 the International Union of Pure and Applied Chemistry IUPAC0 defined the standard boiling point as the temperature of boiling under 1 bar of pressure. The normal boiling point or atmospheric boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at sea level.
Alcohol any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Alcohols may be considered as organic derivatives of water H2O in which a hydrogen atom has been replaced by an alkyl group. Ethanol - Specific Heat C p and C v vs.
Temperature and Pressure - Online calculators figures and tables showing specific heat Cp and Cv of gasous and liquid ethanol at temperatures ranging from -25 to 325 C -10 to 620 F at atmospheric and higher pressure - Imperial and SI Units. 783 NET Optimized code in C The NET optimized code demonstrates the same real-world situation as above but uses modern built-in NET features. The boiling point temperature will be lower if the atmospheric pressure is decreased.
For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at. Ethanol Absolute 200 Proof Molecular Biology Grade Fisher BioReagents.
Absolute 200 Proof Acetone and Isopropyl Alcohol. 995 vv Linear Formula. C 2 H 5 OH.