The atomic mass of Beryllium is 9012 gmol. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.

The atomic mass of Beryllium is 9012 gmol.
Beryllium chloride formula. While beryllium chloride does not ignite easily it may burn with the possible formation of toxic and irritating beryllium oxide fumes and hydrogen chloride. For small fires involving beryllium chloride extinguish with dry chemical CO2 Halon water spray or standard foam and for large fires use water spray fog or standard foam. Beryllium hydroxide created using either the sinter or melt method is then converted into beryllium fluoride or beryllium chloride.
To form the fluoride aqueous ammonium hydrogen fluoride is added to beryllium hydroxide to yield a precipitate of ammonium tetrafluoroberyllate which is heated to 1000 C 1830 F to form beryllium fluoride. Ionic Compound Naming and Formula Writing List 1. Copy this to my account.
E-mail to a friend. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Beryllium hydride systematically named polyberyllane2 and beryllium dihydride is an inorganic compound with the chemical formula BeH 2 n also written BeH 2 n or BeH 2This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it.
Unlike the ionically bonded hydrides of the heavier Group 2 elements beryllium hydride is covalently bonded. Ionic Compounds Naming and Formula Writing. Copy this to my account.
E-mail to a friend. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Today beryllium is primarily obtained from the minerals beryl Be 3 Al 2 SiO 3 6 and bertrandite 4BeO2SiO 2 H 2 O through a chemical process or through the electrolysis of a mixture of molten beryllium chloride BeCl 2 and sodium chloride NaCl.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
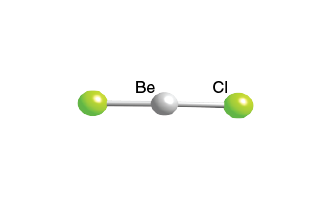
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. The structure of beryllium chloride. As a gas.
Beryllium chloride BeCl 2 is a linear molecule with all three atoms in a straight line. Showing only the outer electrons. Beryllium chloride is known as an electron-deficient compound because it has the two empty orbitals at the bonding level.
As a solid. If it had this same simple structure as a solid you would expect the melting. Beryllium Be - Beryllium with atomic number 4 is a highly toxic bivalent element in the periodic table.
The atomic mass of Beryllium is 9012 gmol. To learn Chemical Properties Uses and FAQs of Beryllium Visit BYJUS for more content. Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen.
It is denoted by the chemical formula KCl and is made up of potassium cations and chloride anions in a 11 ratio. Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell. In its solid form potassium chloride.
Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. 8 water molecules octahydrate. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot.
Show all questions. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structureThe chemical symbol for Beryllium is Be. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust.
The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times. Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions.
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Cations Anions zinc iron II iron III gallium silver lead IV chloride. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3.
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Sodium chloride NaCl and magnesium oxide MgO. The transfer of electrons between metals and non-metals produces charged particles called ions.
Metals lose electrons to produce positve ions called cations. Na Mg 2 Non-metals gain electrons to produce negative ions called anions. Ionic compounds tend to form crystals with high melting temperatures.
Write the metal first and the non-metal second. Use the atomic number to indicate the number of atoms of each type present in the compound. Chemical Formula Nomenclature Practice.
Complete these in lab and on your own time for practice. You should complete this by Sunday. Use the stock form for the transition metals.
Give the formula for the following. Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3.
If the formula is given write down the name and if the name is given write down the formula. Use these pages as a study guide. As a general rule you should know the names and symbols for elements 1-36.
In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Formula to name problems.
1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt. Beryllium makes Be 2 ions. Most of the metals found in group 3 of the periodic table such as indium gallium and aluminum ionize to form 3 cations.
The aluminum cation as seen above is defined as Al 3. Group 6 metalloids and nonmetals such as oxygen tellurium selenium and sulfur produce 2- anions when they ionize. For instance the stable ionized state of oxygen is given as O2-.
When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4. 25 gallium chloride GaCl3 26 sodium hydride NaH 27 beryllium hydroxide BeOH2 28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42.
The negative charge fewer protons than electrons for an anion is shown by a number and minus sign after the formula. If theres just a minus sign it means the charge is minus 1. Review several examples of anions.
Bromide Br-chloride Cl-fluoride F-iodide I-nitride N 3-oxide O 2-sulfide S 2-.