
Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust. Beryllium Go to Course Materials Go to Course PDF pdf icon.
It is utilized as an inert protective gas in autogenous.
Beryllium chemical properties. Structure of the trimeric hydrolysis product of beryllium. Beryllium hydrolysis as a function of pH. Water molecules attached to Be are omitted in this diagram.
A beryllium atom has the electronic configuration He 2s 2. The predominant oxidation state of beryllium is 2. The beryllium atom has lost both of its valence electrons.
Lower oxidation states have been found in. Beryllium Be - Beryllium with atomic number 4 is a highly toxic bivalent element in the periodic table. The atomic mass of Beryllium is 9012 gmol.
To learn Chemical Properties Uses and FAQs of Beryllium Visit BYJUS for more content. Occurrence properties and uses. Beryllium is a steel-gray metal that is quite brittle at room temperature and its chemical properties somewhat resemble those of aluminumIt does not occur free in nature.
Beryllium is found in beryl and emerald minerals that were known to the ancient EgyptiansAlthough it had long been suspected that the two minerals were similar chemical confirmation of. Beryllium hydride systematically named polyberyllane2 and beryllium dihydride is an inorganic compound with the chemical formula BeH 2 n also written BeH 2 n or BeH 2. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it.
Unlike the ionically bonded hydrides of the heavier Group 2 elements beryllium hydride is covalently bonded. Beryllium is used as an alloying agent in the production of beryllium-copper. Thanks to their electrical and thermal conductivity high strenght and hardness non magnetic properties good resistance dimensional stability over a wide temperature range beryllium-copper alloys are used in many applications.
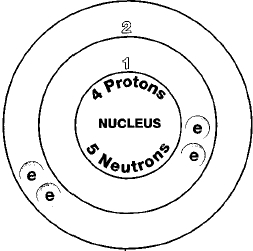
A typical application of beryllium-copper alloys is in the defense and aerospace industries. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structureThe chemical symbol for Beryllium is Be. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust.
The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times. Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structureThe chemical symbol for Beryllium is Be. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust.
The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times. Our beryllium-enhanced products include high strength and high thermal conductivity copper beryllium and nickel beryllium alloys and AlBeMet metal matrix composite. We understand the unique properties of beryllium metal high performance alloys and metal matrix composites and can help you safely maximize its advantages in your products.
Physical Chemical Properties of Tungsten. Tungsten is one of the important strategic resources. Due to its excellent physical and chemical properties tungsten and its alloys are used to manufacture key armor-piercing components that attack various types of armored targets gyro inertial components for satellites and high-temperature anti-ablation components such as rockets combustion.
Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOHIts chemistry is well explored. Reaction with air water and hydrogen. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air.
The alkaline earth metals beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra are a group of chemical elements in the s-block of the periodic table with very similar properties. Somewhat reactive metals at standard temperature and pressure. Chemical properties of vanadium - Health effects of vanadium - Environmental effects of vanadium.
Electronegativity according to Pauling. 61 gcm-3 at 20C. 0074 nm 3.
Electronic shell Ar 3d 3 4s 2. 23723 kilojoules per mole. 52505 kilojoules per mole.
Van der Waals Radius. The primary use of Helium goes in altitudes research and meteorological balloons. It is utilized as an inert protective gas in autogenous.
Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structureThe chemical symbol for Beryllium is Be. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust. The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times.
Beryllium Go to Course Materials Go to Course PDF pdf icon. Cadmium Go to Course Materials Go to Course PDF pdf icon. Cholinesterase Inhibitors Go to Course Materials Go to Course PDF pdf icon.
Chromium Go to Course Materials Go to Course PDF pdf icon. Ethylene Glycol and Propylene Glycol Go to Course Materials Go to Course PDF pdf icon PDF 859 KB. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical designed to prevent confusion arising from different languages and naming systems.
Unknown Period 7 Boiling point. D Density g cm 3 Unknown Atomic number. 112 Relative atomic mass 285 State at 20C.
Solid Key isotopes 285 Cn Electron. Notes on the Melting Point of particular elements. Helium does not solidify at standard pressure.
Value given for diamond form. Value given for yellow phosphorus form. Value given for monoclinic beta form.
Value given for hexagonal gray form. Value given for alpha form. Up to date curated data provided by Mathematicas ElementData.