Helium was first discovered in the sun and its name comes from the ancient Greek word for sun. You may assume that the valences of the elementsthe number of electrons with which an atom will bond or formare those that can be derived by looking at the groups columns of the periodic table.
Reducing agent for beryllium.
Beryllium aluminum compound name. Occurrence properties and uses. Beryllium is a steel-gray metal that is quite brittle at room temperature and its chemical properties somewhat resemble those of aluminumIt does not occur free in nature. Beryllium is found in beryl and emerald minerals that were known to the ancient EgyptiansAlthough it had long been suspected that the two minerals were similar chemical confirmation of.
The objective of the research work was to evaluate the efficiency of three different sampling methods Ghost Wipe micro-vacuum and ChemTest in the recovery of Be dust by assessing. 1 four Be compounds beryllium acetate beryllium chloride beryllium oxide and beryllium aluminium 2 three different surfaces polystyrene glass and aluminium and 3 inter-operator variation. Ionic Compound Naming Chilton Honors Chemistry Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Zinc iron II iron.
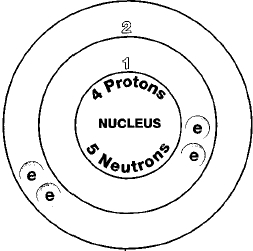
Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structureThe chemical symbol for Beryllium is Be. Beryllium is a hard grayish metal naturally found in mineral rocks coal soil and volcanic dust. The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times.
This Minecraft tutorial explains how to use the features of the Chemistry Update with screenshots and step-by-step instructions. The Chemistry Update was first available in Minecraft Education Edition and has now been added to Minecraft Pocket Edition Windows 10 Xbox One and Nintendo Switch. Beryl ˈ b ɛr əl BERR-əl is a mineral composed of beryllium aluminium cyclosilicate with the chemical formula Be 3 Al 2 Si 6 O 18.
Well-known varieties of beryl include emerald and aquamarineNaturally occurring hexagonal crystals of beryl can be up to several meters in size but terminated crystals are relatively rare. Pure beryl is colorless but it is frequently tinted by. Compound Names and Formulas Worksheet Three For the list on the left name the compound.
For the list on the right give the chemical formula that corresponds to the name Name Formula 1 NaF 13 potassium fluoride 2 K2CO 3 14 ammonium sulfate 3 MgCl 2 15 magnesium iodide 4 BeOH 2 16 copper II sulfite 5 SrS 17 aluminum phosphate 6 Cu 2S 18 lead II nitrite 7 ZnI 2 19 cobalt II. The positive ion cation is written first in the name. The negative ion anion is written second in the name.
When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the. History Aluminium was discovered by Hans Christian Oersted in 1825 at Denmark. From the Latin word alumen meaning alum.
Isotope abundances of aluminium with the most intense signal set to 100. More isotope and NMR data. The prototypical compound is CO 2 which is called carbon dioxide.
The first element shown in the compound is named as the element eg for CO 2 first element is carbon The second element shown in the compound is named according to the anion name ending in -ide eg for CO 2 the second element is named oxide. Ionic compounds tend to form crystals with high melting temperatures. Write the metal first and the non-metal second.
Use the atomic number to indicate the number of atoms of each type present in the compound. The name aluminum is derived from the ancient name for alum potassium aluminum sulphate which was alumen Latin meaning bitter salt. Aluminum was the original name given to the element by Humphry Davy but others called it aluminum and that became the accepted name in Europe.
However in the USA the preferred name was aluminum and when the American Chemical Society debated on the. Aluminium aluminum in American and Canadian English is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals at approximately one third that of steelIt has a great affinity towards oxygen and forms a protective layer of oxide on the surface when exposed to air.
Aluminium visually resembles silver both in its color and. The name for an ionic hydrate is derived by adding a term to the name for the anhydrous meaning not hydrated compound that indicates the number of water molecules associated with each formula unit of the compound. The added word begins with a Greek prefix denoting the number of water molecules see Table 5 and ends with hydrate For example the anhydrous compound copperII.
Alloying agent for aluminum copper and lead. Reducing agent for beryllium. Dehydrating oils Decarburization and desulfurization of iron and its alloys getter in vacuum tubes.
Separation of nitrogen from argon. Reducing agent in preparation of chromium metal powder thorium zirconium and uranium. 8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb 3 N 2 20 AgBr 21 NaBr sodium bromide 22 ScOH 3 scandium III hydroxide 23 V 2 SO 4 3 vanadium III sulfate 24 NH 4 F ammonium fluoride 25 CaCO.
8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb 3 N 2 20 AgBr Ionic Naming Practice Problems - Solutions 1 NaBr sodium bromide 2 ScOH 3 scandium hydroxide 3 V 2 SO 4 3 vanadium III sulfate 4. Name the following ionic compounds. 1 NH4Cl ammonium chloride 2 FeNO33 iron III nitrate 3 TiBr3 titanium III bromide 4 Cu3P copper I phosphide 5 SnSe2 tin IV selenide 6 GaAs gallium arsenide 7 PbSO42 lead IV sulfate 8 BeHCO32 beryllium bicarbonate.
Helium was first discovered in the sun and its name comes from the ancient Greek word for sun. Lithium is the lightest metal so light it floats on water and it reacts with water too. Beryllium is a component of the gemstones beryl emerald and aquamarine.
Li lithium ion Be2 beryllium ion Al3 aluminum ion Na sodium ion Mg2 magnesium ion Ga3 gallium ion K potassium ion Ca2 calcium ion Rb rubidium ion Sr2 strontium ion Cs cesium ion Ba2 barium ion Fr francium ion Ra2 radium ion Ag silver ion Ni2 nickel ion Zn2 zinc ion Cd2 cadmium ion Some metals especially the transition metals with a few exceptions that are printed in blue in. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Origin of the name.
The name is derived from the Latin name for alum alumen meaning bitter salt. The sum of the oxidation states within a compound or ion must equal the overall charge. Atoms of the same element with different numbers of neutrons.
Mode of decay. You may assume that the valences of the elementsthe number of electrons with which an atom will bond or formare those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences the real behavior of electrons is less simple.
In the laboratory small amounts of hydrogen gas may be made by the reaction of calcium hydride with water. CaH 2 2H 2 O CaOH 2 2H 2. This is quite efficient in the sense that 50 of the hydrogen produced comes from water.
Another very convenient laboratory scale experiment follows Boyles early synthesis the reaction of iron filings with dilute.