A Ni b Pd c Pt d Any of these Question. E2 Stereospecific from diastereoisomers.
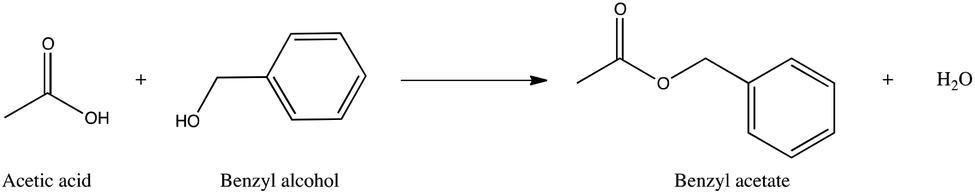
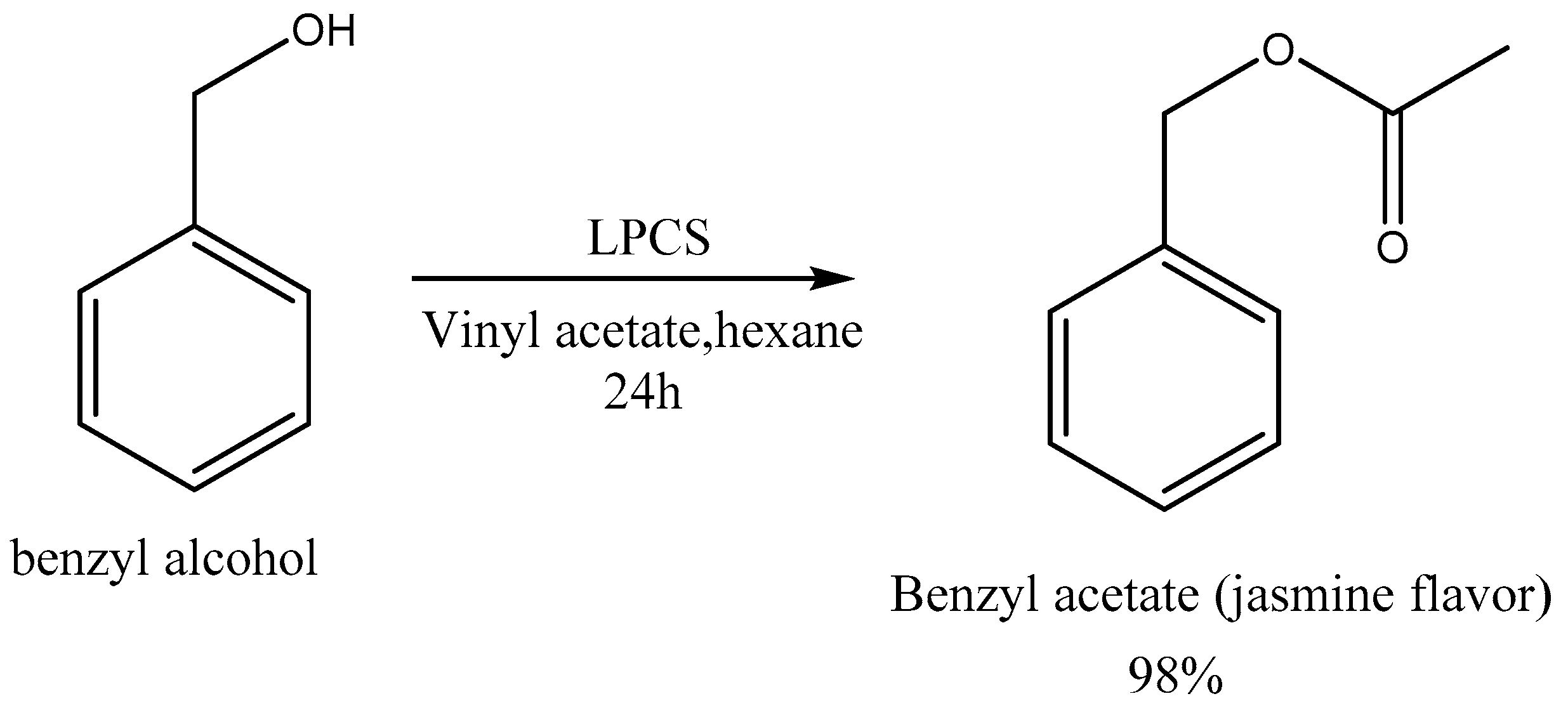
Benzyl acetate was rapidly hydrolyzed to benzyl alcohol and then oxidized to benzoic acid.
Benzyl acetate hydrolysis. Benzyl acetate was rapidly hydrolyzed to benzyl alcohol and then oxidized to benzoic acid. After gavage administration of benzyl acetate in corn oil at 500 mgkg rats and 1000 mgkgmice high benzoic acid plasma concentrations were observed. In contrast much lower benzoic acid plasma concentrations were found after dosed feed administration at about 615 mgkgday for rats and about 850 mg.
Benzyl Ethers in Multi-Step Syntheses. Ammonia pyridine and ammonium acetate were extremely effective as inhibitors of PdC catalyzed benzyl ether hydrogenolysis. While olefin Cbz benzyl ester and azide functionalities were hydrogenated smoothly benzyl ethers were not cleaved.
Sajiki Tetrahedron Lett 1995 36 3465-3468. The safety and effectiveness of Benzyl Alcohol Lotion 5 was demonstrated in two studies of 628 people 6 months of age and older with active head lice infestation. The subjects received two 10-minute treatments of either Benzyl Alcohol Lotion or a topical placebo one week apart.
Fourteen days after the final treatment more than 75 percent of the subjects treated with Benzyl Alcohol. An ester is a chemical compound derived from an acid organic or inorganic in which at least one OH hydroxyl group is replaced by an O alkyl group as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol.
They are important in biology being one of the main classes of lipids and comprising the bulk of animal fats and vegetable. CH 3 CH 2 CH 2 COOCH 2 4 CH 3. CH 3 COOCH 2 7 CH 3.
Which compound has the higher boiling pointCH 3 CH 2 CH 2 CH 2 OH or CH 3 COOCH 3. Which compound is more soluble in watermethyl butyrate or butyric acid. Be used for the enantioselective hydrolysis of acetate esters.
The enantioselective hydrolysis of meso diesters is an important synthetic transformation and racemic esters have been kinetically resolved using lipases. A potentially general method for selectively acylating the primary hydroxyl group of a 12-diol makes use of stannylene acetals as intermediates. Bu2SnO toluene 100 C.
After the electrolysis using a current of 1A at a temperature of 0C 22 faradays per mole of benzyl chloride the remaining benzyl chloride the toluene which is a byproduct of the reduction of benzyl chloride and phenyl-2-propanone both in free form and in the form of its enol acetate are present in the solution. After the DMF has been evaporated off and the residue has been hydrolysed. Containing447 mg benzyl benzoate 20 mg benzyl alcohol and castor oil.
4175156 Estradiol valerate is designated chemically as estra-13510-triene-3 17-diol17β- 17. Benzil is a potent inhibitor of human carboxylesterases enzymes involved in the hydrolysis of carboxylesters and many clinically used drugs. For example with copperII acetate.
PhCOCHOHPh 2 Cu 2 PhCOCOPh 2 H 2 Cu Other suitable oxidizing agents such as nitric acid HNO 3 are used routinely. IronIII chloride FeCl 3 can be used as an inexpensive catalyst for. Benzyl alcohol Benzyl benzoate Benzyl bromoacetate.
For use as preservative only. BHA butylated hydroxyanisole BHT butylated hydroxytoluene Bicyclo221hept-2-ene-6-methyl acrylate 2-Biphenyl diphenyl phosphate Bisbenzoate-O2-propanolatoaluminum CAS Reg. 105442-85-1 For use only as a reactant in the preparation of polyester resins.
S N 2 Reaction. Benzyl Chloride with HS S N 2 Reaction. 2 o Benzyl Chloride with HS S N 2 Mechanism.
E2 to form cyclohexadiene. E2 stereoselective for E alkenes. E2 Stereospecific from diastereoisomers.
E2 Regioselective Elimination to Menthenes A. E2 Regioselective Elimination to Menthenes B. E1cB unimolecular conjuagte base.
Benzyl alcohol is obtained from benzaldehyde by a Fittigs reaction b Cannizzaros reaction c Kolbes reaction d Wurtzs reaction Question. In the reduction R -CHO H 2 RCH 2 OH the catalyst used is. A Ni b Pd c Pt d Any of these Question.
Ethylene reacts with Baeyers reagent to give a ethane b ethyl alcohol c ethylene glycol d None of these. Water hydrolysis will be favorable for 2º 3º-halides. Benzyl C 6 H 5 CH 2 Rapid S N 2 substitution for 1º and 2º-halides.
For 3º-halides a very slow S N 2 substitution or if the nucleophile is moderately basic E2 elimination. In high dielectric ionizing solvents such as water dimethyl sulfoxide. Starch acetate is prepared by acetylation of starch with a mixture of pyridine and acetic acid.
Casting of acetylated starch was realized from solutions of formic acid. The wet strength of the films could be maintained when the acetyl content is sufficient. The starch acetate has a high content of linear amylose and it is consequently more hydrophobic than starch.
By reducing the water. Acetate shaking the tube and applying a sample from the top layer to a TLC plate. However we found that applying a spot directly from the reaction mixture is a lot easier and is suitable for the objective.
Moreover the extraction process takes some time and dilutes the sample making it more difficult for the students to perform the TLC in real time. It is very important that students. This compound can also be prepared via the hydrolysis of benzamide and benzonitrile.
It can also be prepared by oxidizing benzyl chloride or benzyl alcohol or any other derivative of the benzyl group. To learn more about this compound and other aromatic compounds such as pyridine register with BYJUS and download the mobile application on your smartphone. Test your knowledge on benzoic.
Penicillin G is stable against hydrolysis by a variety of beta-lactamases including penicillinases and cephalosporinases and extended spectrum beta-lactamases. Mechanism of action By binding to specific penicillin-binding proteins PBPs located inside the bacterial cell wall penicillin G inhibits the third and last stage of bacterial cell wall synthesis. 3 Application of the.
Vinyl acetate and acrylonitrile as shown in the following equations. Note that in these and many other similar reactions transition metals such as copper and mercury salts are effective catalysts. HCCH HCl HgCl 2 on carbon H 2 CCHCl vinyl chloride.
HCCCH 2 Cl HCl HgCl 2 H 2 CCClCH 2 Cl 23-dichloropropene. HCCH CH. Thiophenols like mercaptans have no action on aluminium.
Benzyl sulphide C 6 H 5 CH 2 2 S is a corrosion inhibitor for aluminium in acid medium. Thioethers also called alcoyl sulphides R 1 SR 2 have no action on aluminium even at high temperatures 100120 C. This applies to allyle sulphide C 6 H 10 S and to.
Amendment of Table AC-1 entry for benzyl chloride filed 12-5-2017. Operative 4-1-2018 Register 2017 No. Appendix to section 5155 Go Back to Article 107 Table of Contents.